Abstract
In recent years, the improvement of curcumin recovery from turmeric by cell and tissue disintegration techniques has been gaining more attention; these emerging techniques were used for a reproducible and robust curcumin extraction process. Additionally, understanding the material characteristics is also needed to choose the optimized technique and appropriate processing parameters. In this review, an outlook about the distribution of different fractions in turmeric rhizomes is reviewed to explain matrix challenges on curcumin extraction. Moreover, the most important part, this review provides a comprehensive summary of the latest studies on ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), enzyme-assisted extraction (EAE), high-pressure-assisted extraction (HPAE), pulsed electric field-assisted extraction (PEFAE), and ohmic heating-assisted extraction (OHAE). Lastly, a detailed discussion about the advantages and disadvantages of emerging techniques will provide an all-inclusive understanding of the food industry’s potential of different available processes.
Similar content being viewed by others
Introduction
Turmeric is an herb that belongs to the ginger family, located mainly in Asia’s tropical climate [1]. The turmeric rhizome has two parts, the main branch in the central and its laterals, axillary branches [2]. From ancient, turmeric was considered a valuable plant with numerous medical benefits and was used as a coloring in the culinary [3]. The most active compound in turmeric is curcumin, which was first isolated in 1815 and first synthesized by Lampe and Milobedeska in 1913 [4]. In recent years, there has been increasing interest in curcumin research, especially related to medical purposes and medicinal benefits considering curcumin to inhibit intestinal cholesterol absorption [5] and to affect LDL oxidation [6], hemostasis, thrombosis, and coagulation [7], type II diabetes [8] or Alzheimer’s disease [9]. Recently, a few reviews comprehensively covered the health perspective of the curcumin [10], curcumin biological activities [11], purification and applications [12], curcumin production and bioavailability [13]. Therefore, these approaches will not be covered in this review. Other recent approaches gaining attention are emerging methods of extracting curcumin from turmeric and adapting processes to be more efficient, faster, sustainable, and eco-friendly. These modern techniques overcome the stability challenges of curcumin with higher efficiency and purity, adapting its application in the food and pharmaceutical industries.
The primary purpose of this review is to give a summarized and comprehensive overview of turmeric rhizome characteristics and the impact of cell disintegration processes on curcumin extraction efficiency. The emerging technologies overcome the matrix challenges of turmeric cells and enhance extraction efficiency; these technologies included ultrasound-assisted extraction [14,15,16], pressurized liquid extraction (PLE) [17, 18], enzyme-assisted extraction (EAE) [19, 20], supercritical fluid extraction (SFE) [21, 22], and microwave-assisted extraction [23, 24].
Turmeric Plant Characteristic
Turmeric Plant Cell Structure
The distribution of oleoresin, curcumin, and starch cell in Curcuma amada, C. aromatica, C. longa, and C. zedoaria are shown in Fig. 1; these components are varied in different species of turmeric. The comparison of the common turmeric rhizome anatomy is shown in Table 1. The turmeric species have common anatomical features like periderm, the outer zone filled with curcumin cells, oil cells, and the inner zone with starch deposition in vascular bundles. Nevertheless, the number of oil and curcumin cells varies in four species; besides, the starch and pectin content is different from species to species [3]. In general, the optimal turmeric cell for efficient extraction has fewer periderm layers and a larger distribution of vascular bundles containing curcumin cells; besides, it should have plenty of curcumin cells and fewer starch granules.
The amount of curcumin cells in C. longa is higher than three other species (Table 1); therefore, the highest number of publications dealing with curcumin extraction is related to this species [1, 25,26,27,28]. There are numerous differences from C. longa to another anatomy of turmeric (Table 1).
The main advantage of C. longa to other types that affects the extraction process is the lower number of periderm layers, the larger distribution of vascular bundles containing curcumin cells, the number of curcumin cells, and the lower number of starch granules.
Different fractions in turmeric could affect curcumin extraction; for example, starch is the barrier that inhibits the diffusion of organic solvent; besides, starch is subjected to swelling and increases the viscosity during thermal treatments. According to Kurmudle et al. [31], α-amylase and glucoamylase are selective and effective enzymes for curcumin extraction. On the other hand, the low content of xylans and cellulose in the turmeric rhizomes do not inhibit the curcumin extraction [31]. The pectin content in the turmeric rhizome is 6.3% w/w [32], and with pectinase treatment, the curcumin recovery could be increased by 53% compared to the untreated sample [33]. In turmeric plant cells, starch and pectin have a noticeable effect on curcumin recovery; numerous studies focused on these components to enhance the efficiency of the extraction process. The efficiency of these approaches will be further discussed in “Evaluation of Structure Modification by Cell Disintegration Index Measurement” and “Effect of Starch Content on the Curcumin Extraction Process.”
Evaluation of Structure Modification by Cell Disintegration Index Measurement
The effect of cell disintegration techniques on turmeric cell structure is shown in Table 2. In order to evaluate the efficiency of pre-treatment on curcumin recovery, cell disintegration index (CDI) was used to reveal the damage of turmeric cells before and after the treatment [33, 41, 42].
The principle of CDI measurement is the difference of impedance measured at the different current frequencies [43]. The difference in material impedance before and after the treatment reveals the damage of the plant cell [44]. According to Shirsath et al. [45], the material matrix is an essential factor affecting the treatment’s efficiency. In the past, mechanical grinding and thermal treatment were used to damage the plant cell. The primary purpose of these techniques was to improve the mass transfer rate due to the plant cell’s increasing permeability [46]. However, these conventional techniques require high-energy usage and long-time treatment [47], which may cause loss of essential components or contamination with undesired compounds. CDI is an important index to compare the efficiency of conventional and non-conventional techniques. According to Luengo et al. [41], an impedance measurement is useful in evaluating plant cell status after the pre-treatment.
In the research of Angersbach et al. [48], the impact of the electroporation induced the formation of pores on the lipid channel but not in the protein channels (Table 2). The frequency area of CDI measurement is in the range of 100 Hz to 10 MHz [44, 49]. According to Ando et al. [43], this range has been widely used to evaluate the cell physiological status of the plant tissue after the pre-treatment. The emerging technologies degrade and disrupt the cell membranes, promoting the diffusion of solvent/curcumin and enhancing the recovery [50].
According to Angersbach et al. [51], the plant disintegration index (\({p}_{0}\)) is described by Eq. (1):
where \({p}_{0}\) is the measured electrical conductivity value and the subscripts i and s refer to the conductivities of untreated (initial) and treated (damaged) tissue, respectively; the subscripts l and h refer to low-frequency and high-frequency range. In theory, \({p}_{0}\) = 0 for an intact tissue and \({p}_{0}\) = 1 for a maximally disintegrated tissue. In most cases, the value of \({p}_{\mathrm{max}}\) was estimated by measurements of freeze-thawed tissue’s electrical conductivity for two rounds [33]. After such treatment, the electrical conductivity of tissue attained its maximal value.
For CDI measurement, as suggested by Angersbach et al. [51], the impedance was evaluated by parallel plate cylindrical electrodes; the distance between two electrodes was 20 mm [48]. The impedance of the material was measured before and after each treatment; the results at low frequency and high frequency were recorded for the calculation [52]. The low-frequency and high-frequency values were chosen in advance, and they varied on the type of materials. In terms of turmeric cell measurement, these values were 5.5 kHz at low frequency and 1.4 MHz at high frequency [33].
The conductivity of the materials increased after the pre-treatment revealed that the plant cell permeabilization increased or the cell membrane was damaged [51]. According to Hayden et al. [53], the damage out the outer plasma membrane of the plant cells will reduce the impedance of materials.
It is essential to understand the effect of the preparation condition on the impedance result. According to Knirsch et al. [54], the impedance of the material could change when it is measured along or across the slice. The different plant cell arrangements could explain this phenomenon in each orientation [54]. Another point that should be considered is that the impedance measurement should take place right after the pre-treatment due to the changing of moisture content could affect the conductivity of the materials [48]. Besides, the temperature of the measurement should be constant due to impedance of plant tissues could be changed by heat [55, 56].
Besides, the particle size may affect the electrical conductivity of materials; with the smaller size, the conductivity tends to increase. In addition, the particle shape and orientation also affect the conductivity of the materials [57]. The disintegration of the cell will decrease the impedance of the materials [54]. Based on the literature, there are also numerous reasons for cell impedance reduction, such as tissue shrinkage, dehydration of material, starch gelatinization by thermal treatment, ion concentration, pH value and fat content of materials, and the viscosity of the environment [54, 58, 59].
When applying the pre-treatment, the cellular membrane can be temporarily damaged (reversible) or permanently damaged (irreversible) [60]. The pre-treatment principle and the pre-treatment conditions will decide the cell damage status. In some cases, the reversible damage cells return to normal after stopping the treatment, such as PEFAE [61] and ohmic heating-assisted extraction (OHAE) [59]. Sometimes, the damaging effect could last for hours after the release of the treatment, such as high-pressure-assisted extraction (HPAE) [48]. Especially, the cell plant destruction was also promoted for hours after the treatment stopped [48]. The CDI measurement offers numerous advantages for evaluating the pre-treatment effect on the turmeric plant cells [33].
Effect of Starch Content on the Curcumin Extraction Process
Starch grains are in turmeric’s inner and outer cores, with triangular, rod, or spindle shapes (Table 1). The starch content in turmeric could be up to 44.15% w/w [62]. Besides, amylose content in isolated turmeric starch was around 48.4% of total starch [63], making the material become swell and burst under the effect of high temperature. These factors could affect the behavior of the aqueous phase during the thermal process. The interaction between starch and water under the impact of H+ ion also improves the starch hydrolysis [64], which could reduce the viscosity of the aqueous phase and increase the diffusion of curcumin during the extraction. Interestingly, turmeric starch starts paste and gelatinization at temperatures higher than 70 °C [65]. So, the techniques with a treatment temperature lower than 70 °C could not efficiently break the starch granule and release amylose.
Recently, numerous studies have removed the turmeric starch before curcumin extraction; enzymes such as α-amylase and amyloglucosidase are used to assist curcumin extraction [31, 66].
Effect of Pectin Content on the Curcumin Extraction Process
The pectin content in turmeric rhizome is around 6.3% w/w [32]. During thermal pre-treatment, pectin could increase the viscosity of the aqueous phase and become a barrier to curcumin diffusion. In addition, when linked to cellulose, pectin provides rigidity and cohesion to cellular walls [67, 68]. Pectinase increases fruit juice exploitation in the food industry by reducing viscosity and improving filtration efficiency [20]. Pectinase has a more significant application in the food industry than amylase; in addition, some previous studies on curcumin extraction showed the efficiency of pectinase on curcumin extraction [33, 66]. In the recent research of Le-Tan et al. [33], curcumin extraction yield reaches 58% when incubated turmeric with pectinase for 1 h prior to extraction (Table 3).
Particularities of Curcumin and Curcumin Stability During Extraction
In turmeric, curcumin is present with demethoxycurcumin (DMC) and bisdemethoxycurcumin (BDMC), which are called curcuminoids; the typical ratio is about 77% of CUR, 17% DMC, and 6% BDMC [69]. The extracts from turmeric consist of all three components (curcuminoids), which need to be further purified for pure curcumin (Fig. 2).
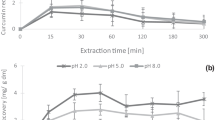
Curcumin is insoluble in water at acidic and neutral pH but dissolves at high alkaline conditions [33]. According to Zheng et al. [70], when pH increases, the deprotonation of hydroxyl groups on curcumin occurs, creating numerous negative charges, increasing hydrophilicity, and the solubility in the aqueous phase. In the research of Rahman et al. [71], curcumin solubility in the aqueous phase is 231 μg/ml at pH 4.0 and increases to 482 μg/ml at pH 8.0. Interestingly, curcumin solubility increases with sodium ions and potassium ions in the aqueous phase [71, 72]. In the report of Le-Tan et al. [37], curcumin recovery increased 25.1% by increasing the pH of the aqueous extraction solvent from 2.0 to 5.0.
Curcumin stability varies depending on the environmental condition. In general, curcumin is stable at high temperatures and in acid conditions but unstable in alkaline conditions and presence of light [72, 73]. The melting point of curcumin is 183 °C [25]. Curcumin is stable at 70 °C when exposed to 10 min in the hydrophilic environment [74]. According to Lestari and Indrayanto [74], curcumin degradation intensively increased at 100 °C. The effect of heat treatment on curcumin stability was studied under different conditions. In the treatments higher than 70 °C for 30 min, the total phenolic content (TPC) in turmeric is significantly reduced [75].
Regarding photo-oxidation, curcumin degradation follows the first-order kinetics [74]. Curcumin photo-degradation begins with the formation of the excited states and singlet oxygen formation, which is responsible for the photodynamic activity of curcumin [76]. According to Lestari and Indrayanto [25], the curcumin stability was different in organic solvents, in which the curcumin half-life degradation followed acetonitrile < chloroform < ethyl acetate < methanol.
Curcumin is not stable in the aqueous phase; the orange-yellow color of curcumin rapidly disappears in the physiological pH conditions [77]. The degradation products of curcumin are vanillin, ferulic acid (FA), and feruloyl methane [78]. In the research of Lestari and Indrayanto [74], vanillin was considered a primary degradation product, and its appearance when incubation was extended.
Curcumin solution changes color in the different pH conditions; at acidic acid, curcumin solution turns red, at pH 1–7, curcumin solution is yellow, and at pH higher than 7.5, the color turns orange-red [72, 79]. The red color at low pH value was due to the presence of curcumin in the protonated form [74], and in the alkaline condition, the formation of degradation products increase the overall absorbance [80]. Interestingly, the curcumin changes solubility in the aqueous phase at different pH conditions, which dissolve at alkaline pH conditions and turn to crystals when pH reduces to acidic value [80]. In a study conducted by Xu et al. [81], over 90% of free curcumin was degraded after 2 days in the pH 4.0 aqueous solution. Curcumin stabled for at least 20 weeks in the organic solvent at 20 °C [78]. Besides, it was found that curcumin was more stable in the dried/solid state [82]. It is important to understand the effect of curcumin degradation under different solvent conditions (pH, temperature, polarity) to design the processing method in the food industry properly. In the research of Gordon et al. [77], the curcumin degradation could be reduced by adding BDMC and DMC into pure curcumin. According to Gordon et al. [77], DMC and BDMC suffer less from autoxidation than curcumin.
Regarding photo-degradation, curcumin strongly absorbs photons in the visible wavelength [83]. Curcumin photo-degradation starts with the formation of the excited states and singlet oxygen creation [33]. Because of these observations, the process of curcumin extraction should adapt with the criteria such as fast, inhibit of light, less contact time with the alkaline condition, and aqueous phase. It is very important to fully understand the properties of curcumin, raw material, and the mechanism of the pre-treatment techniques.
Efficiency of Assisted Extraction Techniques on the Curcumin Recovery Process
Reference Method
In the literature, the conventional techniques for curcumin extraction have been fully reported, such as soxhlet extraction, maceration, and hydrodistillation. These techniques required high temperature, long extraction time, and organic solvent usage. Furthermore, soxhlet has been known as a reference method with a high extraction yield compared with the newly developed methodology [84]. Soxhlet extraction is a conventional and classical method, using organic solvent at boiling temperature to extract target components [85]. Besides, thermal treatment was combined with maceration to increase the curcumin extraction yield. According to Maskooki and Eshtiaghi [61], the thermal treatment with 70–80 °C in 10–20 min could destroy the plant cell membrane. Moreover, Ade-Omowaye et al. [86] demonstrated that a temperature higher than 70 °C is necessary to improve thermal process extraction.
Nonetheless, conventional such as soxhlet, maceration, or hydrodistillation requires prolonged treatment, high-energy consumption, and organic solvent usage [84, 87]. Moreover, these methods could cause the degradation of sensitive compounds. In recent years, cell disruption methods have been an attractive green technology that applied emerging techniques to change the structure of turmeric cells and increase curcumin recovery yield. Recently, these techniques were successfully applied to increase curcumin recovery, such as ultrasound-assisted extraction [45], microwave-assisted extraction [23], enzyme-assisted extraction [88], high-pressure processing [89], pulsed electric field-assisted extraction [90], and ohmic heating-assisted extraction [37] which strongly impact on the turmeric cell membrane to increase the recovery efficiency with minimum effect on the curcumin degradation.
Two possible application scenarios will be considered for some of the technologies: (i) technology applied as pre-treatment followed by the extraction process and (ii) the technology applied simultaneously during the extraction. Each technique has advantages and disadvantages, which are shown in Table 4. Based on these criteria, the researcher could choose an appropriate procedure that suits individual requirements. The mechanism of these techniques and the process efficiency will be further discussed in the following sections.
Ultrasound-Assisted Extraction
Ultrasound-assisted extraction (UAE) has been known as an effective and intensive technique for assisted essential component extraction [99, 100]. The generation of cavitation bubbles with ultra-high temperature and pressure create microturbulence, high-velocity stream circulation inside the sample, particle collisions, and possible rupture of the tissue [15, 16, 101]. These phenomena accelerate solvent penetration and component diffusion [102], increasing extraction efficiency.
Additionally, ultrasound could reduce the particle size of materials under the effect of micron size cavitation [45]. In recent studies, UAE was proven as a save energy method [101] which could create massive effects in a very short time [16]. UAE improved the extraction efficiency by two mechanisms: (a) the penetration of solvent across the tissue and (b) rinsing the component inside the plant cell after weakening the cell wall [103, 104]. Mason [105] reported that ultrasound waves generate intense shear force, microturbulences, and shock waves. During the treatment, extreme motion energy transforms to heat, which induces mass transfer [84, 101]. The obtained temperature and pressure of the microbubbles could reach 5000 K and 1000 atm, respectively [84, 105, 106]. Interestingly, the heating and cooling rate is above 1010 K/s [106]. In recent years, UAE and microwave-assisted extraction (MAE) were the most common non-conventional techniques used for curcumin extraction [12, 14,15,16,17, 19, 45, 92, 107, 108]. Both showed high extraction efficiency, less energy usage, and solvent consumption [12, 13]. The advantages and disadvantages of the UAE are shown in Table 4.
The curcumin extraction by UAE was affected by the material particle size, enzyme content, enzyme activity, the pH condition, incubation temperature, time, and shaking speed [109]. In the report of Shirsath et al. [45], the optimized parameters for curcumin extraction by UAE were 35 °C, solid/liquid ratio of 1:25 (g/ml), US power and frequency at 250 W and 22 kHz, respectively, ethanol as dispersed solvent, and material size was 0.09 mm. The result showed that curcumin recovery was 72% after 1 h, higher than the conventional extraction after 8 h of extraction (62%). Regarding pulsed ultrasound treatment, the curcumin recovery rate was 1.01 g/100 g, and extraction rate constants were 1.46 × 10−2 min−1 (Table 3). In the recent study of Le-Tan et al. [37], the curcumin extraction by ethanol reached 72% with dried turmeric and 41.73% with fresh turmeric (Table 3). These results are similar to the study of Shirsath et al. [45], and the curcumin extraction yield of dried turmeric reached 72% (Table 3).
Regarding pulsed UAE, the total duration and interval time were the main parameters influencing extraction [91]. According to Li et al. [91], curcumin extraction yield decreases if prolonged interval time. In some cases, the high US power could reduce the extraction yield due to the degradation of sensitive compounds [45]. Hence, it is essential to understand the effect of US on the materials and the properties of the desired compounds. Notable, the temperature during the US treatment could not be controlled fully due to the very intense impact from the ultrasonic wave in a short time [110, 111].
Based on the literature, it is vital to understand the limited use of the UAE for natural products. For instance, the high frequencies at 358–850 kHz or high power (> 750 W) could degrade sensitive compounds [112]. Furthermore, the low frequency at 20 kHz combined with high power of 1500 W was also found to degrade anthocyanin [113]. Moreover, the acoustic cavitation could product radicals in the material such as OHAE and H radicals, which may trigger degradation chain reactions of the product [114,115,116,117].
UAE is a promising technique for curcumin-assisted extraction due to numerous advantages over conventional techniques. However, the extract’s quality and drawbacks of the techniques are not fully evaluated [112]. According to Pingret et al. [112], the material matrix and the target extraction components must be carefully considered. Based on recent studies, UAE has been shown as a potential technique to improve curcumin recovery. Compared to conventional extraction methods, the UAE noticeably reduces extraction time energy consumption and improves extraction yield [45]. UAE can be applied in different extraction systems or combined with other methods to increase extraction efficiency [118].
Microwave-Assisted Extraction
MAE has long been known as a rapid and economic-assisted extraction method [119] which requires less energy due to directly heating inside the product [24]. Unlike conventional heating techniques, MAE easily breaks the hydrogen bonds in the materials by electromagnetic effect, resulting in increased solvent penetration and diffusion of soluble compounds into the solvent [107, 108]. According to [120], MAE has been proved a promising assisted extraction technology, which could minimize the thermal degradation of the bioactive compound due to rapid heating mechanism and create fast mass transfer [24]. Yixuan et al. [13] stated that the primary mechanism of MAE is the generation of heat effects by dipole rotation and ion conduction. The transfer of electromagnetic waves induces inside the samples, which significantly improves the mass and heat transfer [120]. Due to microwave irradiation, the cell wall could be degraded by thermal effect, increasing extraction rate and extraction efficiency [92, 121].
Water content in the samples is the prime motivation in the heating process by MAE [108]. The objective of MAE is to heat the moisture in the plant cell, which causes enormous pressure on the cell wall [122]. Based on this effect, the cell wall weakens inside, and cell rupture occurs [123]. Besides, the existing studies suggested that the dielectric susceptibility of the solvent and plant cell matrix play a vital role in MAE utilization [124].
The advantages and disadvantages of MAE are shown in Table 4. The application of MAE in bioactive compound extraction is gaining more attention due to its rapid setup, energy-saving, and feasibility [125]. Besides, MAE could save solvent consumption [126] and, therefore, be considered environmentally friendly technology [12]. Regarding the conventional heating methods, the heat is transferred from the heating wall to the solvent and from the solvent to the material; this process consumes high energy levels and time [122]. However, in terms of MAE, the heated plant cells dramatically increase the temperature due to volumetric heating, leading to cell expansion and cell wall disruption by the internal pressure [119]. As a result, the cell wall opened and released the essential compounds from the plant matrix. Ameer et al. [102] have confirmed the role of radiation waves by MAE, and the rapid heating up of moisture trapped inside the plant matrix could damage the plant matrix. According to Ameer et al. [102], due to the absorption of photonic energy from microwaves, the high temperature was generated from the dehydration of cellulose and created mechanical damage to the cell wall.
In order to decide the optimized parameters for the MAE process, the various levels of microwave power, solvent volume, and treatment time could be investigated [12, 118]. In addition, MAE could be combined with other techniques like ultrasound [127] or enzyme [128] to increase the efficiency of the process. In the report of Yixuan et al. [13], the combination of UAE and EAE increased the extraction rate and antioxidant activity by 2.89% and 83.95%, respectively. In Wakte et al. [92] report, MAE is more efficient than ultrasound and supercritical CO2 (scCO2) in terms of extraction yield and extraction time. Apart from other factors, the material characteristic and features played an essential role in curcumin extraction by MAE [102]; this form includes material moisture, particle sized, sieved, or pre-leached. In the literature, the irradiation time of MAE usually is shorter than 10 min (Table 3). The water soaking could increase the extraction yield of curcumin; the extraction yield was 68.57% w/w [92]. With 800 W of power, the extraction rate was 1.01 g/100 g, and the rate constant was 1.46 × 10−2 min−1 [91]. Notably, in the report of Mandal et al. [34], MAE showed the extraction yield higher than the soxhlet procedure, so the authors considered extraction yield by MAE reached 100% (Table 3).
Enzyme-Assisted Extraction
According to Gligor et al. [20], enzyme-assisted extraction (EAE) has gained higher popularity nowadays in bioactive compound extraction due to highly selective extraction and reduction of environmental hazards. Nevertheless, EAE requires a long incubation time, affecting the sensitive compound because of the long exposure time to oxidation [68]. In order to improve the recovery of curcumin, specific enzymes could be added, such as α-amylase, amyloglucosidase, cellulase, pectinase, and xylanase (Table 3). These enzymes assisted the extraction yield by breaking the plant cell or hydrolyzing the barriers in the plant tissues (polysaccharide, cellulose) [129].
In recent years, EAE revealed the role of improving extraction yield by enabling efficient contact between enzyme and substrate, increasing cell disintegration index, and inducing mass transfer [130,131,132]. According to Rosenthal et al. [129], EAE breaks down the cell wall and exposes the solvent’s intracellular components for recovery. In theory, EAE was considered promising for commercial applications [118] due to its environmental and health-friendly techniques and selective extraction [133].
Numerous factors affect EAE application which include particle size of the materials, the ratio between enzyme and the material, the pH of the mixture, the incubation temperature, time, and shaking method [129,130,131, 133]. In the past few years, some reviews about polyphenol extraction have discussed drawbacks of conventional methods, such as environmental hazards, high consumption of volatile and flammable solvents, high-energy usage, and prolonged exposure time [10, 20, 118, 134]. However, further research on curcumin stability suggested that the soaking of curcumin with water during EAE may degrade the product or promote the oxidation process [11,12,13]. According to Le-Tan et al. [33], the suitable time for curcumin soaking by EAE was 1 h; a longer incubation time could induce the degradation of the extracts.
By breaking the turmeric starch granules, EAE could significantly improve the oleoresin, curcumin, and volatile oil extraction yield. In the research of Kurmudle et al. [66], α-amylase and glucoamylase increased the yields of oleoresin, curcumin, and volatile oil by 22.50%, 31.83%, and 70.00%, respectively.
Regarding EAE, the appropriate enzyme usage depends on the material characteristics [33]. In the report of Leonel et al. [62], the starch content in turmeric is around 44%, while amylose consists 48.4% of total starch [135]. Due to the high amount of starch, α-amylase and amyloglucosidase were frequently chosen [19, 66].
Turmeric contains 9% w/w of cellulose [62]. The study of Kurmudle et al. [66] showed the curcumin extraction yield was 3.44% with the mixture of xylanase and cellulase (Table 3); according to the authors, this yield was low, and the reason could be due to the low contents of xylans and cellulose in the turmeric rhizomes. The pectin content in turmeric is 6.3% w/w [32]. However, in the literature, less study of pectinase has been used for curcumin extraction. In the recent study of Le-Tan et al. [33], pectinase could improve the curcumin extraction yield by 70% (dried turmeric) and 53% (fresh turmeric). According to Le-Tan et al. [33], pectinase could increase the cell disintegration index to 16%. According to Jiang et al. [12], the maximum yield of curcumin extraction by EAE reached 4.1% under the optimum condition. Besides the enzyme type, it is vital to understand the optimum conditions of the enzyme [66]. In the research of Kurmudle et al. [66], α-amylase and glucoamylase had significantly improved the curcumin extraction yield by 31.83% w/w.
Some studies reveal the efficiency of combining EAE with other emerging techniques such as ionic liquid extraction or three-phase partitioning [88]. In the research of Sahne et al. [88], the combination of EAE and N,N-dipropylammonium-N′,N′-dipropylcarbamate (DPCARB) enhanced curcumin extraction yield to 60%.
High-Pressure-Assisted Extraction
High-pressure-assisted extraction (HPAE) is gaining more attention in green extraction technologies because this method requires less treatment time than conventional methods [136] and consumes less energy than other thermal treatments [137]. HPAE disrupts the plant tissue, cellular wall, membrane, and organelles resulting in improved mass transfer and solvent diffusion into the plant structure and soluble compound into the solvent [137]. According to Núñez-Mancilla et al. [138], HPAE could make the cell wall fold and collapse, changing the textural properties due to loss of cellular turgor and wall integrity. The treated materials released the extraction compounds rapidly due to the cell wall collapsing [139]. According to He et al. [140], the diffusion would happen faster than the untreated sample and stop when the equilibrium is reached.
In terms of HPAE, the ground turmeric was mixed with extraction solvent in the antipressure container and then tightly sealed in the HPAE treatment. The container was put in the pressure vessel, and the working pressure increased from atmosphere pressure to desired pressure (from 100 to 1000 Mpa) in a short time (several seconds to minutes) [137]. In some reports, the HPAE could reduce the particle size of materials, improve sedimentation behavior, color, and microstructure, inhibit phase separation, and change the rheological behavior [137, 139, 141, 142]. In addition, HPAE consumes 1% of energy compared to traditional heat reflux extraction [137].
Regarding assisted extraction, the appropriate pressure for HPAE treatment was at 100–1000 MPa at room temperature [137]. According to Prasad et al. [95], 400–600 MPa significantly improved the extraction yield than thermal treatment. The possible mechanism of HPAE pre-treatment on assisted extraction is that the associated proteins on the biomembrane would be solubilized during the HPAE treatment; as a result, the membrane permeability improves [48, 143]. In this regard, a significantly detectable membrane disintegration was found after HPAE treatment at 200 Mpa [48].
HPAE pre-treatment is an environmentally friendly technology for curcumin extraction due to the process being implemented in the closed system, rapid, and use less energy consumption (Table 4). This emerging technology is gaining more attention because it has no adverse effects on human health [136] and environmentally approaches for extracting bioactive components from natural sources [137]. In recent years, HPAE was considered a promising technology to assist the extraction of antioxidant compounds [144]. This approach is gaining more attention because of its high efficiency and rapid treatment [60]. In Plaza et al. [145] report, the total phenolic content was increased 15.46% with the HPAE assisted at 400 MPa/1 min. Furthermore, Prasad et al. [95] revealed that the pressure at 400–600 MPa significantly increased TPC than thermal treatment. The study of Angersbach et al. [48] has also stated that the noticeable effect of HPAE treatment at moderate pressure from 200 to 300 MPa could cause an irreversible effect on the subcellular membrane. In the report of Le-Tan et al. [37], HPAE at 300 MPa increased the cell disintegration index of the turmeric to 72.2% (Table 3), resulting in curcumin recovery reaching 8.38%.
Pulsed Electric Field-Assisted Extraction
Pulsed electric field-assisted extraction (PEFAE) has been frequently used in biology, biotechnology, and medicine [146]. The mechanism of this technique is applying the external electric field to create charge separation, which could induce pore formation on the membrane [52]. According to Zbinden et al. [96], pore formation is induced by the lipid rearrangement within the bilayer due to electrostatic interactions. Moreover, electroporation plays a crucial role in extraction, increasing the membrane permeability and improving extracts’ diffusion [96].
Existing studies suggested that the appropriate treatment time led to saturation of the cell disintegration index (CDI), which means that cell breakdown would not increase when prolonging the treatment time [41]. Furthermore, the food matrix and material characteristics significantly impact the efficiency of the PEFAE treatment [33]. According to Barsotti and Cheftel [147], the reversible membrane rupture can occur if the treatment remains moderate. However, if the electric field strength and treatment time increase, irreversible changes could happen [148]. In addition, the effect of PEFAE treatment also depends on the other characteristic of PEF protocol, such as pulse shape and pulse duration [149].
The advantages and disadvantages of PEFAE are shown in Table 4. PEFAE is a non-thermal treatment with minimal heat effect on bioactive compounds [61]. Besides, the PEFAE duration is ultra-short (in the range of milliseconds to microseconds) [41]; therefore, the exposure time of curcumin to the solvent will be minimized during the pre-treatment-treatment. According to Maskooki and Eshtiaghi [61], PEFAE could limit the changes in food’s sensory and physical properties.
In order to investigate the efficiency of PEFAE treatment with conventional techniques, the comparison may consider different parameters. Regarding time consumption, PEFAE treatment for cell disintegration is ultra-short (≤ 1 min) compared to conventional thermal processing (≥ 10 min) [61]. To completely disintegrate sugar beet cells, the PEFAE treatment consumed 5% energy compared to the conventional thermal treatment, and the diffusion velocity of PEFAE-treated materials was noticeably accelerated [61].
In order to improve the extraction yield, PEFAE could be used as a pre-treatment method before extraction [150]. In the research of Le-Tan et al. [33], the authors used PEFAE as a pre-treatment before rapid shaking extraction by ethanol; the curcumin recovery increased 48.3%. Besides, PEFAE treatment was used to recover valuable compounds from food wastes and food by-products [151,152,153], which is a promising method to recover curcumin from turmeric wastes.
The curcumin extraction yield by ethanol reached 80% (dried turmeric) and 56% (fresh turmeric) (Table 3). Regarding aqueous extraction, curcumin recovery reaches 9.09 g/100 g at pH 5.0.
Ohmic Heating-Assisted Extraction
Ohmic heating is an emerging technology that uses an electric current to internally generate heat based on material electrical resistance [54]. Ohmic heating creates a rapid and uniform heating effect, less thermal damage to the product [154,155,156]. In addition, ohmic heating-assisted extraction (OHAE) is high-energy efficient compared to conventional heating [157]. According to Castro et al. [158], ohmic heating effects depend on the electric current and resistance of the material. In the research of Knirsch et al. [54], OHAE causes electroporation on the membrane of the cells. It was found that the particles in the liquid would be heated up faster than the continuous phase [54]. The previous study of Sastry and Palaniappan [159] also stated that the heating rate on the particles in a liquid is rapid due to the higher electrical resistance of the particles.
The optimized OHAE condition is that the electrical resistance of the particle is equal to the surrounding fluid [160]. Due to its high heat exchange rate, OHAE could be used as high-temperature short time (HTST) or ultra-high-temperature (UHT) treatment on solids or suspended materials [54, 59]. Based on this theory, OHAE has been developed as an alternative method for conventional pre-treatment, which assists in the extraction process [161]. Recently, numerous studies used OHAE as pre-treatment, such as the report of Pereira et al. [161] and Pereira et al. [39]. Regarding curcumin extraction, Le-Tan et al. [37] used OHAE to support the aqueous extraction of curcumin. Furthermore, OHAE is also appropriate for highly viscous products such as mash of fruit and vegetable [162].
The advantages and disadvantages of OHAE are shown in Table 4. OHAE has less effect on the color of materials [98] and provides rapid and uniform heating [163]. While conventional heating transfers the heat through the interfaces of liquid or solid particles, OHAE could create high-temperature hot spots under the effect of electrical current [54]. Based on these advantages, the heating rate of OHAE treatment lasts from a few seconds to a few minutes [164]. In the non-homogenous material, such as a mixture of the solid foods in the liquid phase, the process could be tough to control due to the fluid conductivity fluctuating in a wide range [165]. The heating rate of OHAE in the food material depends on the system’s relative conductivity and volume [166]. Notably, the heating rate in the particle is higher than the continuous phase [159, 166]. According to Palaniappan and Sastry [165], adding the salt solution might enhance the conductance of the materials and affect the efficiency of OHAE treatment. It was also found that blanching could reduce the electric conductivity of the materials due to shrinkage and porosity loss of the material [54]. It is very important to understand the rate of heat generation and the electrical conductivity of materials in OHAE application [167]. Interestingly, the electrical conductivity of food samples could be changed by numerous parameters such as field strength, amount and properties of fat in materials, and cell structure [145, 168, 169]. OHAE has been gaining more attention in recent years [170,171,172]. In curcumin extraction, the most recent research of Le-Tan et al. [37] on curcumin extraction by the aqueous phase showed that the curcumin recovery was 7.84 g/100 g at pH 5.0, while untreated materials had 2.68 g/100 g (Table 3).
To provide a comprehensive understanding of the matrix challenges in the turmeric material and emerging techniques used for curcumin-assisted extraction, an overview picture is seen in Fig. 2.
Outlook
The exploitation of curcumin by emerging technologies is a promising trend. By understanding the characteristic of turmeric rhizome, and the amount and distribution of main fractions in the turmeric tissue and cells, a better understanding of the curcumin extraction process will result. Besides, knowledge about curcumin stability in different conditions will help to enhance the quality of extracts. Furthermore, tailored recovery concepts will have the potential to help in improving the purity of released curcumin. Applying emerging technologies for curcumin recovery may have the advantage of providing a rapid extraction, less energy usage, and high efficiency, which may also result in an improved extraction process for commercial purposes. Future investigations are needed to expand the currently available processes, particularly to enhance current curcumin recovery levels further.
References
Aggarwal BB et al (2006) 10 curcumin—biological and medicinal properties, ed
Agarwal S, Mishra R, Gupta AK, Gupta A (2018) Turmeric: isolation and synthesis of important biological molecules. Synthesis of Medicinal Agents from Plants: Elsevier pp. 105–125
Ravindran P, Babu KN, Sivaraman K (2007) Turmeric: the genus Curcuma. CRC press
Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23(1/A):363–398
Zou J, Zhang S, Li P, Zheng X, Feng D (2018) Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet–fed apolipoprotein E knockout mice. Nutr Res 56:32–40
Assis RP et al (2017) Combined effects of curcumin and lycopene or bixin in yoghurt on inhibition of LDL oxidation and increases in HDL and paraoxonase levels in streptozotocin-diabetic rats. Int J Mol Sci 18(4):332
Keihanian F, Saeidinia A, Bagheri RK, Johnston TP, Sahebkar A (2018) Curcumin, hemostasis, thrombosis, and coagulation. J Cell Physiol 233(6):4497–4511
Su L-Q, Chi H-Y (2017) Effect of curcumin on glucose and lipid metabolism, FFAs and TNF-α in serum of type 2 diabetes mellitus rat models. Saudi journal of biological sciences 24(8):1776–1780
Shabbir U, Rubab M, Tyagi A, Oh D-H (2021) Curcumin and its derivatives as theranostic agents in Alzheimer’s disease: the implication of nanotechnology. Int J Mol Sci 22(1):196
Rauf A, Imran M, Orhan IE, Bawazeer S (2018) Health perspectives of a bioactive compound curcumin: a review. Trends Food Sci Technol 74:33–45
Oglah MK, Mustafa YF, Bashir MK, Jasim MH, Mustafa YF (2020) Curcumin and its derivatives: a review of their biological activities. Syst Rev Pharm 11(3):472
Jiang T, Ghosh R, Charcosset C (2021) Extraction, purification and applications of curcumin from plant materials-a comprehensive review. Trends Food Sci Technol
Yixuan L, Qaria MA, Sivasamy S, Jianzhong S, Daochen Z (2021) Curcumin production and bioavailability: a comprehensive review of curcumin extraction, synthesis, biotransformation and delivery systems. Ind Crops Prod 172:114050
Kimthet C, Wahyudiono, Kanda H, Goto M (2017) Extraction of curcumin from Curcuma longa L. using ultrasound assisted supercritical carbon dioxide. AIP Conf Proc. AIP Publishing LLC, 1840(1):100001
Shirsath SR, Sable SS, Gaikwad SG, Gogate PR (2021) Ultrasound assisted curcumin recovery from Curcuma aromatica: understanding the effect of different operating parameters. Chem Eng Process. p. 108604
Unsal YE, Tuzen M, Soylak M (2019) Ultrasound-assisted ionic liquid-dispersive liquid–liquid of curcumin in food samples microextraction and its spectrophotometric determination. J AOAC Int 102(1):217–221
Chao I-C, Wang C-M, Li S-P, Lin L-G, Ye W-C, Zhang Q-W (2018) Simultaneous quantification of three curcuminoids and three volatile components of curcuma longa using pressurized liquid extraction and high-performance liquid chromatography. Molecules 23(7):1568
Hoff RB, Pizzolato TM (2018) Combining extraction and purification steps in sample preparation for environmental matrices: a review of matrix solid phase dispersion (MSPD) and pressurized liquid extraction (PLE) applications. TrAC, Trends Anal Chem 109:83–96
Sahne F, Mohammadi M, Najafpour GD, Moghadamnia AA (2016) Extraction of bioactive compound curcumin from turmeric (Curcuma longa L.) via different routes: a comparative study. Pakistan J Biotechnol. 13(3):173–180
Gligor O, Mocan A, Moldovan C, Locatelli M, Crișan G, Ferreira IC (2019) Enzyme-assisted extractions of polyphenols–a comprehensive review. Trends Food Sci Technol 88:302–315
Khanam S (2018) Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J Clean Prod 188:816–824
Lv GP, Hu DJ, Zhou YQ, Zhang QW, Zhao J, Li SP (2018) Preparation and application of standardized typical volatile components fraction from turmeric (Curcuma longa L.) by supercritical fluid extraction and step molecular distillation. Molecules 23(7):1831
Doldolova K, Bener M, Lalikoğlu M, Aşçı YS, Arat R, Apak R (2021) Optimization and modeling of microwave-assisted extraction of curcumin and antioxidant compounds from turmeric by using natural deep eutectic solvents. Food Chem 353:129337
Popescu V et al (2019) Sustainable and cleaner microwave-assisted dyeing process for obtaining eco-friendly and fluorescent acrylic knitted fabrics. J Clean Prod 232:451–461
Lestari ML, Indrayanto G. (2014) Profiles Drug Subst Excip Relat Methodol 39:113-204
Metzler M, Pfeiffer E, Schulz SI, Dempe JS (2013) Curcumin uptake and metabolism. Biofactors. 39(1):14-20. https://doi.org/10.1002/biof.1042.
Aggarwal BB, Sundaram C, Malani N, Ichikawa H (2007) Curcumin: the Indian solid gold. Adv Exp Med Biol 595:1–75. https://doi.org/10.1007/978-0-387-46401-5_1
Vogel H, Pelletier J (1815) Curcumin-biological and medicinal properties. J Pharma 2(50):24–29
Li S, Yuan W, Deng G, Wang P, Yang P, Aggarwal B (2011) Chemical composition and product quality control of turmeric (Curcuma longa L.)
Govindarajan V, Stahl WH (1980) Turmeric—chemistry, technology, and quality. Critical Reviews in Food Science Nutrition 12(3):199–301
Kurmudle NN, Bankar SB, Bajaj IB, Bule MV, Singhal RS (2011) Enzyme-assisted three phase partitioning: a novel approach for extraction of turmeric oleoresin. Process Biochem 46(1):423–426
Harsha MR, Prakash SV, Dharmesh SM (2016) Modified pectic polysaccharide from turmeric (Curcuma longa): A potent dietary component against gastric ulcer. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2015.11.043
Le-Tan H, Fauster T, Vladic J, Gerhardt T, Haas K, Jaeger H (2021) Application of Emerging Cell Disintegration Techniques for the Accelerated Recovery of Curcuminoids from Curcuma longa. Applied Sciences. 11(17):8238 [Online] Available: https://www.mdpi.com/2076-3417/11/17/8238
Mandal V, Mohan Y, Hemalatha S (2008) Microwave assisted extraction of curcumin by sample–solvent dual heating mechanism using Taguchi L9 orthogonal design. Journal of pharmaceutical biomedical analysis 46(2):322–327
Mandal V, Mohan Y, Hemalatha S (2007) Microwave assisted extraction—an innovative and promising extraction tool for medicinal plant research. Pharmacogn Rev 1(1):7–18
Puri M, Sharma D, Barrow CJ (2012) Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol 30(1):37–44
Le-Tan H, Fauster T, Haas K, Jaeger H (2022) Aqueous Extraction of Curcuminoids from Curcuma longa: Effect of Cell Disintegration Pre-treatment and Extraction Condition. Food Bioprocess Technol 15(6):1359-73
Alexandre E, Araújo P, Duarte MF, de Freitas V, Pintado M, Saraiva JA (2017) High-pressure assisted extraction of bioactive compounds from industrial fermented fig by-product. J Food Sci Technol 54(8):2519–2531
Pereira RN et al (2016) Effects of ohmic heating on extraction of food-grade phytochemicals from colored potato. LWT 74:493–503
Rodrigues RM et al (2015) Influence of moderate electric fields on gelation of whey protein isolate. Food Hydrocolloids 43:329–339
Luengo E, Martínez JM, Álvarez I, Raso J (2016) Effects of millisecond and microsecond pulsed electric fields on red beet cell disintegration and extraction of betanines. Ind Crops Prod 84:28–33
Maskooki A, Eshtiaghi MN (2012) Impact of pulsed electric field on cell disintegration and mass transfer in sugar beet. Food Bioproducts Processing 90(3):377–384
Ando Y, Mizutani K, Wakatsuki N (2014) Electrical impedance analysis of potato tissues during drying. J Food Eng 121:24–31
Donsì F, Ferrari G, Pataro G (2010) Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Engineering Reviews 2(2):109–130
Shirsath S, Sable S, Gaikwad S, Sonawane S, Saini D, Gogate P (2017) Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: effect of different operating parameters. Ultrason Sonochem 38:437–445
Toepfl S, Mathys A, Heinz V, Knorr D (2006) Potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Rev Intl 22(4):405–423
Puértolas E, Luengo E, Álvarez I, Raso J (2012) Improving mass transfer to soften tissues by pulsed electric fields: fundamentals and applications. Annu Rev Food Sci Technol 3:263–282
Angersbach A, Heinz V, Knorr D (2002) Evaluation of process-induced dimensional changes in the membrane structure of biological cells using impedance measurement. Biotechnol Prog 18(3):597–603
Damez J-L, Clerjon S, Abouelkaram S, Lepetit J (2007) Dielectric behavior of beef meat in the 1–1500 kHz range: simulation with the Fricke/Cole–Cole model. Meat Sci 77(4):512–519
Puri M, Sharma D, Barrow CJ (2012) Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol 30(1):37–44. https://doi.org/10.1016/j.tibtech.2011.06.014
Angersbach A, Heinz V, Knorr D (1999) Electrophysiological model of intact and processed plant tissues: cell disintegration criteria. Biotechnol Prog 15(4):753–762
Fauster T et al (2018) Impact of pulsed electric field (PEF) pretreatment on process performance of industrial French fries production. J Food Eng 235:16–22
Hayden R, Moyse C, Calder F, Crawford D, Fensom D (1969) Electrical impedance studies on potato and alfalfa tissue. J Exp Bot 20(2):177–200
Knirsch MC, Dos Santos CA, de Oliveira Soares AA, Penna TC (2010) Ohmic heating–a review. Trends Food Sci Technol 21(9):436-41
Repo T, Zhang M, Ryyppö A, Vapaavuori E, Sutinen S (1994) Effects of freeze-thaw injury on parameters of distributed electrical circuits of stems and needles of Scots pine seedlings at different stages of acclimation. J Exp Bot 45(6):823–833
Zhang M, Willison J (1992) Electrical impedance analysis in plant tissues: the effect of freeze-thaw injury on the electrical properties of potato tuber and carrot root tissues. Can J Plant Sci 72(2):545–553
Leizerson S, Shimoni E (2005) Effect of ultrahigh-temperature continuous ohmic heating treatment on fresh orange juice. J Agric Food Chem 53(9):3519–3524
Praporscic I, Lebovka N, Ghnimi S, Vorobiev E (2006) Ohmically heated, enhanced expression of juice from apple and potato tissues. Biosys Eng 93(2):199–204
Imai T, Uemura K, Ishida N, Yoshizaki S, Noguchi A (1995) Ohmic heating of Japanese white radish Rhaphanus sativus L. Int J Food Sci Technol 30(4):461–472
Barba FJ, Zhu Z, Koubaa M, Sant’Ana AS, Orlien V (2016) Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: a review. Trends Food Sci Technol 49:96–109
Maskooki A, Eshtiaghi MN (2012) Impact of pulsed electric field on cell disintegration and mass transfer in sugar beet. Food Bioprod Process 90(3):377–384
Leonel M, Sarmento SB, Cereda MP (2003) New starches for the food industry: Curcuma longa and Curcuma zedoaria. Carbohyd Polym 54(3):385–388
Kuttigounder D, Lingamallu JR, Bhattacharya S (2011) Turmeric powder and starch: selected physical, physicochemical, and microstructural properties. J Food Sci 76(9):C1284-91. https://doi.org/10.1111/j.1750-3841.2011.02403.x
Moreschi S, Leal J, Braga M, Meireles M (2006) Ginger and turmeric starches hydrolysis using subcritical water+ CO2: the effect of the SFE pre-treatment. Braz J Chem Eng 23(2):235–242
Kuttigounder D, Lingamallu JR, Bhattacharya S (2011) Turmeric powder and starch: selected physical, physicochemical, and microstructural properties. J Food Sci 76(9):C1284-91
Kurmudle N, Kagliwal LD, Bankar SB, Singhal RS (2013) Enzyme-assisted extraction for enhanced yields of turmeric oleoresin and its constituents. Food Biosci 3:36–41
Danalache F, Mata P, Alves VD, Moldão-Martins M (2018) Enzyme-assisted extraction of fruit juices. Fruit Juices pp. 183-200
Muniglia L, Claisse N, Baudelet PH, Ricochon G (2014) Enzymatic aqueous extraction (EAE). Alter Solvent Nat Products Extract. Springer pp 167–204
Horosanskaia E, Yuan L, Seidel-Morgenstern A, Lorenz H (2020) Purification of curcumin from ternary extract-similar mixtures of curcuminoids in a single crystallization step. Crystals 10(3):206
Zheng B, Zhang X, Lin H, McClements DJ (2019) Loading natural emulsions with nutraceuticals using the pH-driven method: formation & stability of curcumin-loaded soybean oil bodies. Food Funct 10(9):5473–5484
Rahman SM, Telny TC, Ravi TK, Kuppusamy S (2009) Role of surfactant and pH in dissolution of Curcumin. Indian J Pharm Sci 71(2):139–142. https://doi.org/10.4103/0250-474X.54280
Le-Tan H, Fauster T, Haas K, Jaeger H (2022) Evaluation of the synergistic effect of plant-based components on the stability of curcuminoid emulsion. Eur Food Res Technol
Kiamahalleh MV, Najafpour-Darzi G, Rahimnejad M, Moghadamnia AA, Kiamahalleh MV (2016) High performance curcumin subcritical water extraction from turmeric (Curcuma longa L.). J Chromatogr B. 1022:191-8
Lestari ML, Indrayanto G (2014) Curcumin. Profiles Drug Subst Excip Relat Methodol 39:113–204. https://doi.org/10.1016/B978-0-12-800173-8.00003-9
Prathapan A, Lukhman M, Arumughan C, Sundaresan A, Raghu K (2009) Effect of heat treatment on curcuminoid, colour value and total polyphenols of fresh turmeric rhizome. Int J Food Sci Technol 44(7):1438–1444
Chignell CF, Bilskj P, Reszka KJ, Motten AG, Sik RH, Dahl TA (1994) Spectral and photochemical properties of curcumin. Photochem Photobiol 59(3):295–302
Gordon ON, Luis PB, Ashley RE, Osheroff N, Schneider C (2015) Oxidative transformation of demethoxy-and bisdemethoxycurcumin: products, mechanism of formation, and poisoning of human topoisomerase IIα. Chem Res Toxicol 28(5):989–996
Schieffer GW (2002) Pressurized liquid extraction of curcuminoids and curcuminoid degradation products from turmeric (Curcuma longa) with subsequent HPLC assays. J Liq Chromatogr Relat Technol 25(19):3033–3044
Nguyen VT, Huynh TM, Nguyen TN, Le TH (2021) Enhancing the stability of synthesized curcumin by spray-drying microencapsulation with soy lecithin and gum Arabic. Br J Chem Eng 38(3):563-72
Kharat M, Du Z, Zhang G, McClements DJ (2017) Physical and chemical stability of curcumin in aqueous solutions and emulsions: impact of pH, temperature, and molecular environment. J Agric Food Chem 65(8):1525–1532
Xu G, Wang C, Yao P (2017) Stable emulsion produced from casein and soy polysaccharide compacted complex for protection and oral delivery of curcumin. Food Hydrocolloids 71:108–117
Khurana A, Ho C-T (1988) High performance liquid chromatographic analysis of curcuminoids and their photo-oxidative decomposition compounds in Curcuma longa L. J Liq Chromatogr 11(11):2295–2304
Jankun J et al (2016) Determining whether curcumin degradation/condensation is actually bioactivation. Int J Mol Med 37(5):1151–1158
Azmir J et al (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117(4):426–436
Al Juhaimi F, Özcan MM, Ghafoor K, Babiker EE, Hussain S (2018) Comparison of cold-pressing and soxhlet extraction systems for bioactive compounds, antioxidant properties, polyphenols, fatty acids and tocopherols in eight nut oils. J Food Sci Technol 55(8):3163-73
Ade-Omowaye B, Angersbach A, Taiwo K, Knorr D (2001) Use of pulsed electric field pre-treatment to improve dehydration characteristics of plant based foods. Trends Food Sci Technol 12(8):285–295
Le TH et al (2017) Combination of whey protein and carbohydrate for microencapsulation of pumpkin (Cucurbita spp.) seed oil by spray-drying. Int Food Res J 24(3)
Sahne F, Mohammadi M, Najafpour GD, Moghadamnia AA (2017) Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Industrial crops and products. 95:686-94
Choi Y, Kim W, Lee JS, Youn SJ, Lee H, Baik MY (2020) Enhanced antioxidant capacity of puffed turmeric (Curcuma longa L.) by high hydrostatic pressure extraction (HHPE) of bioactive compounds. Foods. 9(11):1690
Poompavai S, Sree VG, Sundararajan R (2019) Enhanced extraction of bioactive compounds from natural herbs by pulsed electric field method. Proc 2019 Ann Meeting Electrostat Am
Li M, Ngadi MO, Ma Y (2014) Optimisation of pulsed ultrasonic and microwave-assisted extraction for curcuminoids by response surface methodology and kinetic study. Food Chem 165:29–34
Wakte PS, Sachin B, Patil A, Mohato D, Band T, Shinde D (2011) Optimization of microwave, ultra-sonic and supercritical carbon dioxide assisted extraction techniques for curcumin from Curcuma longa. Sep Purif Technol 79(1):50–55
Wakte PS, Sachin B, Patil A, Mohato D, Band T, Shinde D (2011) Optimization of microwave, ultra-sonic and supercritical carbon dioxide assisted extraction techniques for curcumin from Curcuma longa. Separation purification technology 79(1):50–55
Sahne F, Mohammadi M, Najafpour GD, Moghadamnia AA (2017) Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Industrial crops and products. 95:686-94
Prasad KN, Yang E, Yi C, Zhao M, Jiang Y (2009) Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. Innov Food Sci Emerg Technol 10(2):155–159
Zbinden MDA et al (2013) Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol Bioeng 110(6):1605–1615
Ramaswamy HS, Marcotte M, Sastry S, Abdelrahim K (2014) Ohmic heating in food processing. CRC press
Varghese KS, Pandey M, Radhakrishna K, Bawa A (2014) Technology, applications and modelling of ohmic heating: a review. J Food Sci Technol 51(10):2304–2317
Vilkhu K, Mawson R, Simons L, Bates D (2008) Applications and opportunities for ultrasound assisted extraction in the food industry—a review. Innovative Food Science Emerging Technologies 9(2):161–169
Mason TJ, Paniwnyk L, Lorimer J (1996) The uses of ultrasound in food technology. Ultrason Sonochem 3(3):S253–S260
Kadam SU, Tiwari BK, Álvarez C, O’Donnell CP (2015) Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci Technol 46(1):60–67
Ameer K, Shahbaz HM, Kwon JH (2017) Green extraction methods for polyphenols from plant matrices and their byproducts: a review. Comprehensive Reviews in Food Science and Food Safety 16(2):295–315
Toma M, Vinatoru M, Paniwnyk L, Mason TJ (2001) Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason Sonochem 8(2):137–142
Jadhav D, Rekha B, Gogate PR, Rathod VK (2009) Extraction of vanillin from vanilla pods: a comparison study of conventional soxhlet and ultrasound assisted extraction. J Food Eng 93(4):421–426
Mason TJ (1996) Adv Sonochem. Elsevier
Suslick KS, Doktycz S, Flint E (1990) On the origin of sonoluminescence and sonochemistry. Ultrasonics 28(5):280–290
Hadi BJ, Sanagi MM, Aboul-Enein HY, Ibrahim WA, Jamil S, Mu’azu MA (2015) Microwave-assisted extraction of methyl β-cyclodextrin-complexed curcumin from turmeric rhizome oleoresin. Food Anal Methods. 8(10):2447-56
Bener M, Özyürek M, Güçlü K, Apak R (2016) Optimization of microwave-assisted extraction of curcumin from Curcuma longa L.(Turmeric) and evaluation of antioxidant activity in multi-test systems. Rec Nat Prod. 10(5):542
Yusoff MM, Gordon MH, Ezeh O, Niranjan K (2016) Aqueous enzymatic extraction of Moringa oleifera oil. Food Chem 211:400–408
Lou Z, Wang H, Zhang M, Wang Z (2010) Improved extraction of oil from chickpea under ultrasound in a dynamic system. J Food Eng 98(1):13–18
Balachandran S, Kentish S, Mawson R, Ashokkumar M (2006) Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason Sonochem 13(6):471–479
Pingret D, Fabiano-Tixier A-S, Chemat F (2013) Degradation during application of ultrasound in food processing: a review. Food Control 31(2):593–606
Tiwari BK, O'donnell CP, Cullen PJ (2009) Effect of non thermal processing technologies on the anthocyanin content of fruit juices. Trends Food Sci Technol 20(3-4):137-45
Riesz P, Berdahl D, Christman C (1985) Free radical generation by ultrasound in aqueous and nonaqueous solutions. Environ Health Perspect 64:233–252
Makino K, Mossoba MM, Riesz P (1983) Chemical effects of ultrasound on aqueous solutions. Formation of hydroxyl radicals and hydrogen atoms, The Journal of physical chemistry 87(8):1369–1377
Czechowska-Biskup R, Rokita B, Lotfy S, Ulanski P, Rosiak JM (2005) Degradation of chitosan and starch by 360-kHz ultrasound. Carbohyd Polym 60(2):175–184
Huang H et al (2019) Ultrasonic impact on viscosity and extraction efficiency of polyethylene glycol: a greener approach for anthocyanins recovery from purple sweet potato. Food Chem 283:59–67
Saini RK, Keum Y-S (2018) Carotenoid extraction methods: a review of recent developments. Food Chem 240:90–103
Hiranvarachat B, Devahastin S (2014) Enhancement of microwave-assisted extraction via intermittent radiation: extraction of carotenoids from carrot peels. J Food Eng 126:17–26
Praveen MA, Parvathy KK, Balasubramanian P, Jayabalan R (2019) An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci Technol 92:46–64
Dhobi M, Mandal V, Hemalatha S (2009) Optimization of microwave assisted extraction of bioactive flavonolignan-silybinin. J Chem Metrol. 3(1):13
Bagade SB, Patil M (2021) Recent advances in microwave assisted extraction of bioactive compounds from complex herbal samples: a review. Crit Rev Anal Chem 51(2):138–149
Abdel-Aal ESM, Akhtar H, Rabalski I, Bryan M (2014) Accelerated, microwave-assisted, and conventional solvent extraction methods affect anthocyanin composition from colored grains. J Food Sci 79(2):C138–C146
Delazar A, Nahar L, Hamedeyazdan S, Sarker SD (2012) Microwave-assisted extraction in natural products isolation. Nat Prod Isolation. pp 89-115
Das I, Arora A (2018) Alternate microwave and convective hot air application for rapid mushroom drying. J Food Eng 223:208–219
Mena-García A, Ruiz-Matute AI, Soria AC, Sanz ML (2019) Green techniques for extraction of bioactive carbohydrates. TrAC, Trends Anal Chem 119:115612
Garcia-Vaquero M, Ummat V, Tiwari B, Rajauria G (2020) Exploring ultrasound, microwave and ultrasound–microwave assisted extraction technologies to increase the extraction of bioactive compounds and antioxidants from brown macroalgae. Mar Drugs 18(3):172
Hu B et al (2020) Oil extraction from tiger nut (Cyperus esculentus L.) using the combination of microwave-ultrasonic assisted aqueous enzymatic method-design, optimization and quality evaluation. J Chromatogr A.1627:461380
Rosenthal A, Pyle D, Niranjan K (1996) Aqueous and enzymatic processes for edible oil extraction. Enzyme Microb Technol 19(6):402–420
Joana Gil‐Chávez G et al (2013) Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci 12(1):5–23
Roselló-Soto E et al (2016) Application of non-conventional extraction methods: toward a sustainable and green production of valuable compounds from mushrooms. Food Engineering Reviews 8(2):214–234
Azwanida N (2015) A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med Aromat Plants 4(196):2167–2412
Sowbhagya H, Chitra V (2010) Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit Rev Food Sci Nutr 50(2):146–161
Khan SA, Aslam R, Makroo HA (2019) High pressure extraction and its application in the extraction of bio-active compounds: a review. J Food Process Eng 42(1):e12896
Kuttigounder D, Lingamallu JR, Bhattacharya S (2011) Turmeric powder and starch: selected physical, physicochemical, and microstructural properties. J Food Sci 76(9):C1284–C1291
Pereira R, Vicente A (2010) Environmental impact of novel thermal and non-thermal technologies in food processing. Food Res Int 43(7):1936–1943
Huang H-W, Hsu C-P, Yang BB, Wang C-Y (2013) Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci Technol 33(1):54–62
Núñez-Mancilla Y, Vega-Gálvez A, Pérez-Won M, Zura L, García-Segovia P, Di Scala K (2014) Effect of osmotic dehydration under high hydrostatic pressure on microstructure, functional properties and bioactive compounds of strawberry (Fragaria vesca). Food Bioprocess Technol 7(2):516–524
Xi J, Shen D, Li Y, Zhang R (2011) Ultrahigh pressure extraction as a tool to improve the antioxidant activities of green tea extracts. Food Res Int 44(9):2783–2787
He Z, Huang Y, Li H, Qin G, Wang T, Yang J (2012) Effect of high-pressure treatment on the fatty acid composition of intramuscular lipid in pork. Meat Sci 90(1):170–175
Kubo MTK, Augusto PE, Cristianini M (2013) Effect of high pressure homogenization (HPH) on the physical stability of tomato juice. Food Res Int 51(1):170–179
Augusto PE, Ibarz A, Cristianini M (2013) Effect of high pressure homogenization (HPH) on the rheological properties of tomato juice: viscoelastic properties and the Cox-Merz rule. J Food Eng 114(1):57–63
Kato M, Hayashi R (1999) Effects of high pressure on lipids and biomembranes for understanding high-pressure-induced biological phenomena. Biosci Biotechnol Biochem 63(8):1321–1328
Barba FJ, Terefe NS, Buckow R, Knorr D, Orlien V (2015) New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review, Food Research International 77:725–742
Plaza L, Sánchez-Moreno C, De Ancos B, Elez-Martínez P, Martín-Belloso O, Cano MP (2011) Carotenoid and flavanone content during refrigerated storage of orange juice processed by high-pressure, pulsed electric fields and low pasteurization. LWT-Food Science and Technology 44(4):834–839
Miklavcic D (2019) Handbook of electroporation. Springer
Barsotti L, Cheftel J (1999) Food processing by pulsed electric fields. II. Biological aspects. Food Rev Int 15(2):181–213
Barba FJ, Zhu Z, Koubaa M, Sant'Ana AS, Orlien V, Technology (2016) Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: a review. Trends Food Sci Technol 49:96–109
Blahovec J, Vorobiev E, Lebovka N (2017) Pulsed electric fields pretreatments for the cooking of foods. Food engineering reviews 9(2):71–81
Vorobiev E, Lebovka NI (2006) Extraction of intercellular components by pulsed electric fields. InPulsed electric fields technology for the food industry. Springer pp. 153-193
Roselló-Soto E et al (2015) Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: non-conventional methods for the recovery of high-added value compounds. Trends Food Sci Technol 45(2):296–310
Yuan L et al (2015) Genetic analysis of the RAB39B gene in Chinese Han patients with Parkinson’s disease. Neurobiol Aging. 36(10):2907, e11–2907, e12
Galanakis CM (2012) Recovery of high added-value components from food wastes: conventional, emerging technologies and commercialized applications. Trends Food Sci Technol 26(2):68–87
Sun H, Kawamura S, Himoto J-I, Itoh K, Wada T, Kimura T (2008) Effects of ohmic heating on microbial counts and denaturation of proteins in milk. Food science and technology research 14(2):117–123
Vicente A, Castro Id, Teixeira J (2006) Ohmic Heating Food Process
Ruan R, Ye X, Chen P, Doona C, Taub I, Center NS (2001) Ohmic Heating Therm Technol Food Process pp. 165–241
Saberian H, Hamidi‐Esfahani Z, Ahmadi Gavlighi H, Banakar A, Barzegar M (2018) The potential of ohmic heating for pectin extraction from orange waste. J Food Process Preserv 42(2):e13458
Castro I, Teixeira JA, Salengke S, Sastry SK, Vicente AA (2004) Ohmic heating of strawberry products: electrical conductivity measurements and ascorbic acid degradation kinetics. Innov Food Sci Emerg Technol 5(1):27–36
Sastry SK, Palaniappan S (1992) Mathematical modeling and experimental studies on ohmic heating of liquid-particle mixtures in a static heater 1. J Food Process Eng 15(4):241–261
Wang W-C, Sastry SK (1993) Salt diffusion into vegetable tissue as a pretreatment for ohmic heating: electrical conductivity profiles and vacuum infusion studies. J Food Eng 20(4):299–309
Pereira RN, Coelho MI, Genisheva Z, Fernandes JM, Vicente AA, Pintado ME (2020) Using ohmic heating effect on grape skins as a pretreatment for anthocyanins extraction. Food Bioprod Process 124:320–328
Mannozzi C et al (2018) Role of thermal and electric field effects during the pre-treatment of fruit and vegetable mash by pulsed electric fields (PEF) and ohmic heating (OH). Innov Food Sci Emerg Technol 48:131–137
Kaur R, Gul K, Singh A (2016) Nutritional impact of ohmic heating on fruits and vegetables—a review. Cogent Food Agric 2(1):1159000
Jun S, Sastry S (2005) Modeling and optimization of ohmic heating of foods inside a flexible package. J Food Process Eng 28(4):417–436
Palaniappan S, Sastry SK (1991) Electrical conductivities of selected solid foods during ohmic heating 1. J Food Process Eng 14(3):221–236
Sarang S, Sastry S, Gaines J, Yang T, Dunne P (2007) Product formulation for ohmic heating: blanching as a pretreatment method to improve uniformity in heating of solid–liquid food mixtures. J Food Sci 72(5):E227–E234
Bozkurt H, Icier F (2010) Electrical conductivity changes of minced beef–fat blends during ohmic cooking. J Food Eng 96(1):86–92
Wang WC, Sastry SK (1997) Changes in electrical conductivity of selected vegetables during multiple thermal treatments 1. J Food Process Eng 20(6):499–516
Halden K, De Alwis A, Fryer P (1990) Changes in the electrical conductivity of foods during ohmic heating. Int J Food Sci Technol 25(1):9–25
Tunç MT, Odabaş Hİ (2021) Single-step recovery of pectin and essential oil from lemon waste by ohmic heating assisted extraction/hydrodistillation: a multi-response optimization study. Innov Food Sci Emerg Technol 74:102850
Cabas BM, Icier F (2021) Ohmic heating–assisted extraction of natural color matters from red beetroot. Food Bioprocess Technol pp. 1–16
Al-Hilphy AR, Al-Musafer AM, Gavahian M (2020) Pilot-scale ohmic heating-assisted extraction of wheat bran bioactive compounds: effects of the extract on corn oil stability. Food Res Int 137:109649
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). This study was supported by the University of Natural Resources and Life Sciences Vienna (BOKU) in the frame of the OeAD Grant No. Ref. MPC-2020–01134.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Le-Tan, H., Jaeger, H. Impact of Cell Disintegration Techniques on Curcumin Recovery. Food Eng Rev 14, 655–672 (2022). https://doi.org/10.1007/s12393-022-09319-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-022-09319-x