Abstract
Freshwater edible algae have been consumed as a source of food and nutrition for humans as well as animals for centuries. Among the several bioactive components in freshwater algae, polysaccharides are considered the most important due to their numerous health benefits. Freshwater edible algae polysaccharides (FEAPs) are complex carbohydrate molecules that are considered a promising source of natural functional ingredients in the food industry owing to their potential functional properties and health benefits such as antioxidant, antimicrobial, antidiabetic, antitumor, and prebiotic activities. They are also known for their high molecular weight, high water-holding capacity, and excellent gelling and thickening properties, making them a natural alternative to synthetic thickeners and stabilizers. Therefore, this review aims to present an overview of the importance of FEAP, their extraction and purification techniques, monosaccharide composition, structural properties, and applications. The complexity of their extraction and purification procedures and limited research on their properties can sometimes limit their practical applications. Despite these challenges, there are opportunities for expanding FEAP production by developing sustainable production systems, processing technologies and increasing consumer awareness and thereby creating more opportunities in the food, pharmaceutical, cosmetic, and biotechnology industries as a natural source of bioactive compounds, which contributes to sustainable food security and environmental sustainability. The present review focused on the prominence of polysaccharides isolated from fresh water algae, novel technologies used for their extraction, purification, structural characterization, and their potential application in food industries.
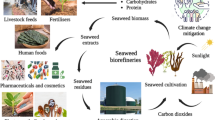
Graphical Abstract

Similar content being viewed by others
References
Costa JAV, Lucas BF, Alvarenga AGP, Moreira JB, de Morais MG (2021) Microalgae polysaccharides: an overview of production, characterization, and potential applications. Polysaccharides 2(4):759–772. https://doi.org/10.3390/polysaccharides2040046
Tan JS, Lee SY, Chew KW, Lam MK, Lim JW, Ho SH, Show PL (2020) A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 11(1):116–129
Show PL, Tan JS, Lee SY, Chew KW, Lam MK, Lim JW, Ho S-H (2020) A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered. https://doi.org/10.1080/21655979.2020.1711626
Koyande AK, Chew KW, Rambabu K, Tao Y, Chu D-T, Show P-L (2019) Microalgae: a potential alternative to health supplementation for humans. Food Sci Hum Wellness 8:16–24
Kumar BR, Mathimani T, Sudhakar MP, Rajendran K, Nizami A-S, Brindhadevi K, Pugazhendhi A (2021) A state of the art review on the cultivation of algae for energy and other valuable products: application, challenges, and opportunities. Renew Sustain Energy Rev 138:110649. https://doi.org/10.1016/j.rser.2020.110649
Ross IL, Shah S, Hankamer B, Amiralian N (2021) Microalgal nanocellulose opportunities for a circular bioeconomy. Trends Plant Sci. https://doi.org/10.1016/jtplants202105004
El-Naggar NE-A, Hussein MH, Shaaban-Dessuuki SA, Dalal SR (2020) Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci Rep. https://doi.org/10.1038/s41598-020-59945-w
Russell C, Rodriguez C, Yaseen M (2021) High-value biochemical products & applications of freshwater eukaryotic microalgae. Sci Total Environ 809:151111
Sudhakar MP, Kumar BR, Mathimani T, Arunkumar K (2019) A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J Clean Prod 228:1320–1333
Lee XJ, Ong HC, Gan YY, Chen WH, Mahlia TMI (2020) State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers Manag 210:112707
Beaumont M, Tran R, Vera G et al (2021) Hydrogel-forming algae polysaccharides: from seaweed to biomedical applications. Biomacromol 22(3):1027–1052. https://doi.org/10.1021/acs.biomac.0c01406
Safari R, Raftani Amiri Z, Esmaeilzadeh KR (2020) Antioxidant and antibacterial activities of C-phycocyanin from common name Spirulina platensis. Iran J Fish Sci 19(4):1911–1927
Rahman A, Kumar S, Nawaz T (2020) Biosynthesis of nanomaterials using algae. In: Microalgae cultivation for biofuels production. pp 265–279. https://doi.org/10.1016/b978-0-12-817536-1.00017-5
Mirzaie S, Tabarsa M, Safavi M (2021) Effects of extracted polysaccharides from a Chlorella vulgaris biomass on expression of interferon-γ and interleukin-2 in chicken peripheral blood mononuclear cells. J Appl Phycol 33:409–418. https://doi.org/10.1007/s10811-020-02301-2
Mohammed AS, Naveed M, Jost N (2021) Polysaccharides; classification, chemical properties, and future perspective applications in fields of pharmacology and biological medicine (A review of current applications and upcoming potentialities). J Polym Environ 29:2359–2371
Mutanda T, Naidoo D, Bwapwa JK, Anandraj A (2020) Biotechnological applications of microalgal oleaginous compounds: current trends on microalgal bioprocessing of products. Front Energy Res 8:598803
Ravindran R, Rajauria G (2021) Carbohydrates derived from microalgae in the food industry. In: Cultured microalgae for the food industry. pp 127–146. https://doi.org/10.1016/B978-0-12-821080-2.00007-1
Fu W, Nelson DR, Mystikou A, Daakour S, Salehi-Ashtiani K (2019) Advances in microalgal research and engineering development. Curr Opin Biotechnol 59:157–164
Silvello MADC, Gonçalves IS, Azambuja SPH, Costa SS, Silva PGP, Santos LO, Goldbeck R (2022) Microalgae-based carbohydrates: a green innovative source of bioenergy. Bioresour Technol 344:126304
Prybylski N, Toucheteau C, El Alaoui H, Bridiau N, Maugard T, Abdelkafi S, Michaud P (2020) Bioactive polysaccharides from microalgae. In: Jacob-Lopes E, Maroneze MM, Queiroz MI, Zepka LQ (eds) Handbook of microalgae-based processes and products. Fundamentals and advances in energy, food, feed, fertilizer, and bioactive compounds. Academic Press, Cambridge, pp 533–571. https://doi.org/10.1016/B978-0-12-818536-0.00020-8
de Sousa e Silva A, de Magalhães WT, Moreira LM, Rocha MVP, Bastos AKP (2018) Microwave-assisted extraction of polysaccharides from Arthrospira (Spirulina) platensis using the concept of green chemistry. Algal Res 35:178–184. https://doi.org/10.1016/j.algal.2018.08.015
Nigam S, Singh R, Bhardwaj SK et al (2022) Perspective on the therapeutic applications of algal polysaccharides. J Polym Environ 30:785–809. https://doi.org/10.1007/s10924-021-02231-1
Tounsi L, Hentati F, Hlima HB, Barkallah M, Smaoui S, Fendri I, Michaud P, Abdelkafi S (2022) Microalgae as feedstock for bioactive polysaccharides. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2022.08.206
Yi Z, Su Y, Brynjolfsson S, Olafsdóttir K, Fu W (2021) Bioactive polysaccharides and their derivatives from microalgae: biosynthesis, applications, and challenges. Stud Nat Prod Chem 71:67–85
Paterson S, Gómez-Cortés P, de la Fuente MA, Hernández-Ledesma B (2023) Bioactivity and digestibility of microalgae Tetraselmis sp. and Nannochloropsis sp. as basis of their potential as novel functional foods. Nutrients 15(2):477
Perveen S, Yang L, Zhou S, Feng B, Xie X, Zhou Q, Qian D, Wang C, Yin F (2021) β-1,3-Glucan from Euglena gracilis as an immunostimulant mediates the antiparasitic effect against Mesanophrys sp. on hemocytes in marine swimming crab (Portunus trituberculatus). Fish Shellfish Immunol 114:28–35
Morais MG, Santos TD, Moraes L, Vaz BS, Morais EG, Costa JA (2022) Exopolysaccharides from microalgae: production in a biorefinery framework and potential applications. Bioresour Technol Rep 18:101006
Darwish R, Gedi MA, Eakpetch P, Assaye H, Zaky AS, Gray DA (2020) Chlamydomonas reinhardtii is a potential food supplement with the capacity to outperform Chlorella and Spirulina. Appl Sci 10(19):6736. https://doi.org/10.3390/app10196736
Mularczyk M, Michalak I, Marycz K (2020) Astaxanthin and other nutrients from Haematococcus pluvialis—multifunctional applications. Mar Drugs 18(9):459. https://doi.org/10.3390/md18090459
Wang B, Liu Q, Huang Y, Yuan Y, Ma Q, Du M, Cai T, Cai Y (2018) Extraction of polysaccharide from Spirulina and evaluation of its activities. Evid Based Complement Alternat Med. https://doi.org/10.1155/2018/3425615
de Jesus CS, de Jesus Assis D, Rodriguez MB, Menezes Filho JA, Costa JA, de Souza Ferreira E, Druzian JI (2019) Pilot-scale isolation and characterization of extracellular polymeric substances (EPS) from cell-free medium of Spirulina sp. LEB-18 cultures under outdoor conditions. Int J Biol Macromol 124:1106–1114
Madhubalaji CK, Mudaliar SN, Chauhan VS et al (2021) Evaluation of drying methods on nutritional constituents and antioxidant activities of Chlorella vulgaris cultivated in an outdoor open raceway pond. J Appl Phycol 33:1419–1434. https://doi.org/10.1007/s10811-020-02355-2
Verspreet J, Soetemans L, Gargan C, Hayes M, Bastiaens L (2021) Nutritional profiling and preliminary bioactivity screening of five micro-algae strains cultivated in Northwest Europe. Foods 10(7):1516. https://doi.org/10.3390/foods10071516
Hildebrand G, Poojary MM, O’Donnell C et al (2020) Ultrasound-assisted processing of Chlorella vulgaris for enhanced protein extraction. J Appl Phycol 32:1709–1718. https://doi.org/10.1007/s10811-020-02105-4
Chia SR, Chew KW, Zaid HFM, Chu D-T, Tao Y, Show PL (2019) Microalgal protein extraction from Chlorella vulgaris FSP-E using triphasic partitioning technique with sonication. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2019.00396
Krishnan S, Ghani NA, Aminuddin NF, Quraishi KS, Azman NS, Cravotto G, Leveque J-M (2019) Microwave-assisted lipid extraction from Chlorella vulgaris in water with 0.5%–2.5% of imidazolium based ionic liquid as additive. Renew Energy. https://doi.org/10.1016/j.renene.2019.12.063
Seon G, Joo HW, Kim YJ, Park J, Chang YK (2018) Hydrolysis of lipid-extracted Chlorella vulgaris by simultaneous use of solid and liquid acids. Biotechnol Prog. https://doi.org/10.1002/btpr.2729
Molino A, Iovine A, Casella P, Mehariya S, Chianese S, Cerbone A et al (2018) Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int J Environ Res Public Health 15(11):2436. https://doi.org/10.3390/ijerph15112436
Villaró S, Morillas-España A, Acién G, Lafarga T (2022) Optimisation of operational conditions during the production of Arthrospira platensis using pilot-scale raceway reactors, protein extraction, and assessment of their techno-functional properties. Foods 11(15):2341
Sánchez-Laso J, Piera A, Vicente G, Bautista LF, Rodríguez R, Espada JJ (2021) A successful method for phycocyanin extraction from Arthrospira platensis using [Emim] [EtSO4] ionic liquid. Biofuels Bioprod Biorefin. https://doi.org/10.1002/bbb.2275
Casazza AA, Spennati E, Converti A, Busca G (2020) Production of carbon-based biofuels by pyrolysis of exhausted Arthrospira platensis biomass after protein or lipid recovery. Fuel Process Technol 201:106336. https://doi.org/10.1016/j.fuproc.2020.106336
da Silva MET, de Paula Correa K, Martins MA, da Matta SLP, Martino HSD, dos Reis Coimbra JS (2020) Food safety, hypolipidemic and hypoglycemic activities, and in vivo protein quality of microalga Scenedesmus obliquus in Wistar rats. J Funct Foods 65:103711
Jaeschke DP, Mercali GD, Marczak LDF, Müller G, Frey W, Gusbeth C (2019) Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.03.035
Chaiklahan R, Chirasuwan N, Loha V, Tia S, Bunnag B (2018) Stepwise extraction of high-value chemicals from Arthrospira (Spirulina) and an economic feasibility study. Biotechnol Rep. https://doi.org/10.1016/j.btre.2018.e00280
da Costa Menestrino B, Sala L, Costa JAV et al (2021) Magnetic fields exhibit a positive impact on lipid and biomass yield during phototrophic cultivation of Spirulina sp. Bioprocess Biosyst Eng 44:2087–2097. https://doi.org/10.1007/s00449-021-02585-9
Mahima J, Sundaresh RK, Gopinath KP, Rajan PSS, Arun J, Kim S-H, Pugazhendhi A (2021) Effect of algae (Scenedesmus obliquus) biomass pre-treatment on bio-oil production in hydrothermal liquefaction (HTL): biochar and aqueous phase utilization studies. Sci Total Environ 778:146262. https://doi.org/10.1016/j.scitotenv.2021.146
El-Baz FK, Abdo SM, El-Sayed DAA, Mostafa MA, Elsherif HMR, Safaa HM, Abdon AS (2021) Application of defatted Scenedesmus obliquus biomass for broilers’ nutrition. Braz J Poult Sci. https://doi.org/10.1590/1806-9061-2020-1366
Ferreira AS, Ferreira SS, Correia A, Vilanova M, Silva TH, Coimbra MA, Nunes C (2020) Reserve, structural and extracellular polysaccharides of Chlorella vulgaris: a holistic approach. Algal Res 45:101757. https://doi.org/10.1016/j.algal.2019.101757
Alves JLF, Da Silva JCG, Costa RL et al (2019) Investigation of the bioenergy potential of microalgae Scenedesmus acuminatus by physicochemical characterization and kinetic analysis of pyrolysis. J Therm Anal Calorim 135:3269–3280. https://doi.org/10.1007/s10973-018-7506-2
da Silveira Rossi RA, Barbosa JM, de Souza Barrozo MA, Vieira LG (2021) Catalytic solar hydropyrolysis of the Chlamydomonas reinhardtii microalgae. Biomass Bioenergy 152:106183
Metsoviti MN, Papapolymerou G, Karapanagiotidis IT, Katsoulas N (2019) Comparison of growth rate and nutrient content of five microalgae species cultivated in greenhouses. Plants 8(8):279. https://doi.org/10.3390/plants8080279
Andrade LA, Barrozo MAS, Vieira LGM (2018) Catalytic solar pyrolysis of microalgae Chlamydomonas reinhardtii. Sol Energy 173:928–938. https://doi.org/10.1016/j.solener.2018.08.035
Aligata AJ, Tryner J, Quinn JC et al (2019) Effect of microalgae cell composition and size on responsiveness to ultrasonic harvesting. J Appl Phycol 31:1637–1649. https://doi.org/10.1007/s10811-018-1682-0
Xu L, Cheng X, Wang Q (2018) Enhanced lipid production in Chlamydomonas reinhardtii by co-culturing with Azotobacter chroococcum. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00741
Nutautaitė M, Vilienė V, Racevičiūtė-Stupelienė A, Bliznikas S, Karosienė J, Koreivienė J (2021) Freshwater Cladophora glomerata biomass as promising protein and other essential nutrients source for high quality and more sustainable feed production. Agriculture 11(7):582
Michalak I, Mironiuk M, Marycz K (2018) A comprehensive analysis of biosorption of metal ions by macroalgae using ICP-OES, SEM-EDX and FTIR techniques. PLoS ONE 13(10):e0205590. https://doi.org/10.1371/journal.pone.0205590
Nikkhah H, Tavasoli A, Jafarian S (2020) Investigating the influence of acid washing pretreatment and Zn/activated biochar catalyst on thermal conversion of Cladophora glomerata to value-added bio-products. Energy Convers Manag 225:113392. https://doi.org/10.1016/j.enconman.2020.11339
Unpaprom Y, Whangchai N, Prasongpol P (2020) Antibacterial, antifungal properties and chemical composition of freshwater macroalage, Cladophora glomerata. J Biol Med Open Access 1(1):107
Santos B, da Conceição DP, Corrêa DO et al (2022) Changes in gene expression and biochemical composition of Haematococcus pluvialis grown under different light colors. J Appl Phycol 34:729–743. https://doi.org/10.1007/s10811-022-02696-0
Zhu Y, Zhao X, Zhang X, Liu H (2019) Extraction, structural and functional properties of Haematococcus pluvialis protein after pigment removal. Int J Biol Macromol 140:1073–1083. https://doi.org/10.1016/j.ijbiomac.2019.08
Colusse GA, Duarte MER, de Carvalho JC, Noseda MD (2019) Media effects on laboratory scale production costs of Haematococcus pluvialis biomass. Bioresour Technol Rep 7:100236. https://doi.org/10.1016/j.biteb.2019.100236
Zhao Y, Yue C, Ding W, Li T, Xu J-W, Zhao P, Yu X (2018) Butylated hydroxytoluene induces astaxanthin and lipid production in Haematococcus pluvialis under high-light and nitrogen-deficiency conditions. Bioresour Technol 266:315–321. https://doi.org/10.1016/j.biortech.2018.06.1
Molino A, Mehariya S, Di Sanzo G, Larocca V, Martino M, Leone GP et al (2020) Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: role of key parameters, technological achievements and challenges. J CO2 Utiliz 36:196–209. https://doi.org/10.1016/j.jcou.2019.11.014
Severo IA, Dias RR, do Nascimento TC, Deprá MC, Maroneze MM, Zepka LQ, Jacob-Lopes E (2022) Microalgae-derived polysaccharides: potential building blocks for biomedical applications. World J Microbiol Biotechnol 38(9):150
Singh H, Varanasi JL, Banerjee S, Das D (2019) Production of carbohydrate enrich microalgal biomass as a bioenergy feedstock. Energy. https://doi.org/10.1016/j.energy.2019.116039
Roja K, Ruben Sudhakar D, Anto S, Mathimani T (2019) Extraction and characterization of polyhydroxyalkanoates from marine green alga and cyanobacteria. Biocatal Agric Biotechnol 22:101358. https://doi.org/10.1016/j.bcab.2019.101358
Zhuang D, He N, Khoo KS, Ng EP, Chew KW, Ling TC (2021) Application progress of bioactive compounds in microalgae on pharmaceutical and cosmetics. Chemosphere 291:132932
Tang W, Liu D, Yin J-Y, Nie S-P (2020) Consecutive and progressive purification of food-derived natural polysaccharide: based on material, extraction process and crude polysaccharide. Trends Food Sci Technol. https://doi.org/10.1016/j.tifs.2020.02.015
Sosa-Hernández JE, Escobedo-Avellaneda Z, Iqbal HM, Welti-Chanes J (2018) State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 23(11):2953
Bordoloi A, Goosen N (2020) Green and integrated processing approaches for the recovery of high-value compounds from brown seaweeds, vol 95. Elsevier Ltd., Amsterdam
Patel AK, Vadrale AP, Singhania RR et al (2022) Algal polysaccharides: current status and future prospects. Phytochem Rev. https://doi.org/10.1007/s11101-021-09799-5
Ray B, Ali I, Jana S, Mukherjee S, Pal S, Ray S, Schütz M, Marschall M (2022) Antiviral strategies using natural source-derived sulfated polysaccharides in the light of the COVID-19 pandemic and major human pathogenic viruses. Viruses 14(1):35
Zollmann M, Robin A, Prabhu M, Polikovsky M, Gillis A, Greiserman S, Golberg A (2019) Green technology in green macroalgal biorefineries. Phycologia 58(5):516–534
García-Pérez JS, Cuéllar-Bermúdez SP, Arévalo-Gallegos A et al (2020) Influence of supercritical CO2 extraction on fatty acids profile, volatile compounds and bioactivities from Rosmarinus officinalis. Waste Biomass Valoriz 11:1527–1537. https://doi.org/10.1007/s12649-018-0408-5
Di Caprio F, Altimari P, Pagnanelli F (2021) Ultrasound-assisted extraction of carbohydrates from microalgae. Chem Eng Trans 86:25–30
Yu M, Chen M, Gui J, Huang S, Liu Y, Shentu H et al (2019) Preparation of Chlorella vulgaris polysaccharides and their antioxidant activity in vitro and in vivo. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.06.22
Huang G, Chen F, Yang W, Huang H (2021) Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci Technol 109:564–568. https://doi.org/10.1016/j.tifs.2021.01.038
Mirzadeh M, Arianejad MR, Khedmat L (2020) Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: a review. Carbohydr Polym 229:115421
Gharibzahedi SM, Marti-Quijal FJ, Barba FJ, Altintas Z (2022) Current emerging trends in antitumor activities of polysaccharides extracted by microwave- and ultrasound-assisted methods. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2022.01.088
Lefebvre T, Destandau E, Lesellier E (2020) Selective extraction of bioactive compounds from plants using recent extraction techniques: a review. J Chromatogr A. https://doi.org/10.1016/j.chroma.2020.461770
Bagade SB, Patil M (2021) Recent advances in microwave assisted extraction of bioactive compounds from complex herbal samples: a review. Crit Rev Anal Chem 51(2):138–149
Yirgu Z, Leta S, Hussen A, Khan MM, Aragaw T (2021) Optimization of microwave-assisted carbohydrate extraction from indigenous Scenedesmus sp. grown in brewery effluent using response surface methodology. Heliyon 7(5):e07115. https://doi.org/10.1016/j.heliyon.2021.e07115
Gligor O, Mocan A, Moldovan C, Locatelli M, Crișan G, Ferreira IC (2019) Enzyme-assisted extractions of polyphenols—a comprehensive review. Trends Food Sci Technol 88:302–315
Nadar SS, Rao P, Rathod VK (2018) Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: a review. Food Res Int 108:309–330. https://doi.org/10.1016/j.foodres.2018.03.006
Bernaerts TM, Gheysen L, Foubert I, Hendrickx ME, Van Loey AM (2019) The potential of microalgae and their biopolymers as structuring ingredients in food: a review. Biotechnol Adv 37(8):107419
Echave J, Fraga-Corral M, Garcia-Perez P, Popović-Djordjević J, Avdović EH, Radulović M, Xiao J, Prieto MA, Simal-Gandara J (2021) Seaweed protein hydrolysates and bioactive peptides: extraction, purification, and applications. Mar Drugs 19(9):500
Dobrinčić A, Balbino S, Zorić Z, Pedisić S, Bursać Kovačević D, Elez Garofulić I, Dragović-Uzelac V (2020) Advanced technologies for the extraction of marine brown algal polysaccharides. Mar Drugs 18(3):168
Andrade TA, Hamerski F, López Fetzer DE, Roda-Serrat MC, Corazza ML, Norddahl B, Errico M (2021) Ultrasound-assisted pressurized liquid extraction of anthocyanins from Aronia melanocarpa pomace. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2021.119290
Zhou J, Wang M, Berrada H, Zhu Z, Grimi N, Barba FJ (2022) Pulsed electric fields (PEF), pressurized liquid extraction (PLE) and combined PEF+ PLE process evaluation: effects on Spirulina microstructure, biomolecules recovery and triple TOF-LC-MS-MS polyphenol composition. Innov Food Sci Emerg Technol 77:102989
Khadhraoui B, Ummat V, Tiwari BK, Fabiano-Tixier AS, Chemat F (2021) Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason Sonochem 76:105625
Poojary MM, Barba FJ, Aliakbarian B, Donsì F, Pataro G, Dias DA, Juliano P (2016) Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar Drugs 14(11):214
Yousefi M, Rahimi-Nasrabadi M, Pourmortazavi SM, Wysokowski M, Jesionowski T, Ehrlich H, Mirsadeghi S (2019) Supercritical fluid extraction of essential oils. Trends Anal Chem. https://doi.org/10.1016/j.trac.2019.05.038
Zhao T, Yan X, Sun L, Yang T, Hu X, He Z, Liu F, Liu X (2019) Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci Technol 91:354–361
Fomo G, Madzimbamuto TN, Ojumu TV (2020) Applications of nonconventional green extraction technologies in process industries: challenges, limitations and perspectives. Sustainability 12(13):5244
Rocha CMR, Genisheva Z, Ferreira-Santos P, Rodrigues R, Vicente AA, Teixeira JA, Pereira RN (2018) Electric field-based technologies for valorization of bioresources. Bioresour Technol 254(March):325–339. https://doi.org/10.1016/j.biortech.2018.01.068
AlYammahi J, Rambabu K, Thanigaivelan A et al (2022) Advances of non-conventional green technologies for phyto-saccharides extraction: current status and future perspectives. Phytochem Rev. https://doi.org/10.1007/s11101-022-09831-2
Muhamad II, Zulkifli N, Selvakumaran S A/P, Lazim NAM (2019) Bioactive algal-derived polysaccharides: multi-functionalization, therapeutic potential and biomedical applications. Curr Pharm Des. https://doi.org/10.2174/138161282566619061815
Liu J, Wu S-Y, Chen L, Li Q-J, Shen Y-Z, Jin L, Zhang X, Chen P-C, Wu M-J, Choi J et al (2019) Different extraction methods bring about distinct physicochemical properties and antioxidant activities of Sargassum fusiforme fucoidans. Int J Biol Macromol. https://doi.org/10.1007/s00018-019-03071-y
Monguió-Tortajada M, Gálvez-Montón C, Bayes-Genis A, Roura S, Borràs FE (2019) Extracellular vesicle isolation methods: rising impact of size-exclusion chromatography. Cell Mol Life Sci 76(12):2369–2382
Mourão PA, Vilanova E, Soares PA (2018) Unveiling the structure of sulfated fucose-rich polysaccharides via nuclear magnetic resonance spectroscopy. Curr Opin Struct Biol 50:33–41
Kronusová O, Kaštánek P, Koyun G, Kaštánek F, Brányik T (2022) Factors influencing the production of extracellular polysaccharides by the green algae Dictyosphaerium chlorelloides and their isolation, purification, and composition. Microorganisms 10(7):1473
Balti R, Le Balc’h R, Brodu N, Gilbert M, Le Gouic B, Le Gall S, Sinquin C, Massé A (2018) Concentration and purification of Porphyridium cruentum exopolysaccharides by membrane filtration at various cross-flow velocities. Process Biochem 74:175–184
Guo R, Chen M, Ding Y, Yang P, Wang M, Zhang H, He Y, Ma H (2022) Polysaccharides as potential anti-tumor biomacromolecules—a review. Front Nutr. https://doi.org/10.3389/fnut.2022.838179
Laroche C (2022) Exopolysaccharides from microalgae and cyanobacteria: diversity of strains, production strategies, and applications. Mar Drugs 20(5):336. https://doi.org/10.3390/md20050336
Gaignard C, Laroche C, Pierre G, Dubessay P, Delattre C, Gardarin C, Gourvil P, Probert I, Dubuffet A, Michaud P (2019) Screening of marine microalgae: investigation of new exopolysaccharide producers. Algal Res 44:101711
Canelli G, Murciano Martínez P, Austin S, Ambühl ME, Dionisi F, Bolten CJ, Mathys A (2021) Biochemical and morphological characterization of heterotrophic Crypthecodinium cohnii and Chlorella vulgaris cell walls. J Agric Food Chem 69(7):2226–2235. https://doi.org/10.1021/acs.jafc.0c05032
Chen C, Tang T, Shi Q, Zhou Z, Fan J (2022) The potential and challenge of microalgae as promising future food sources. Trends Food Sci Technol. https://doi.org/10.1016/j.tifs.2022.06.016
Attia MS, El-Sayyad GS, Saleh SS et al (2019) Spirulina platensis-polysaccharides promoted green silver nanoparticles production using gamma radiation to suppress the expansion of pear fire blight-producing Erwinia amylovora. J Clust Sci 30:919–935. https://doi.org/10.1007/s10876-019-01550-7
Ma H, Xiong H, Zhu X, Ji C, Xue J, Li R et al (2019) Polysaccharide from Spirulina platensis ameliorates diphenoxylate-induced constipation symptoms in mice. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.04.209
Zhang J, Liu L, Chen F (2019) Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.05.11
Patil L, Kaliwal BB (2019) Microalga Scenedesmus bajacalifornicus BBKLP-07, a new source of bioactive compounds with in vitro pharmacological applications. Bioprocess Biosyst Eng 42(6):979–994
Kokarakis EJ, Nazos TT, Mavroudakis L, Stratigakis NC, Sfendourakis GP, Lioudaki S, Spyros A, Pergantis SA, Ghanotakis DF (2022) Structural and physicochemical characterization of an aminosugar-rich exopolysaccharide isolated from a Chlorella sp. Algal Res 68:102881
Periyannan R, Subramanian P, Ravichandran A, Manoharan V, Meyyanathan E, Thangapandi M et al (2019) Isolation and structural characterization of sulfated polysaccharides from Spirulina platensis and its bioactive potential: in vitro antioxidant, antibacterial activity and Zebra fish growth and reproductive performance. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2019.09.02
Song H, He M, Gu C, Wei D, Liang Y, Yan J, Wang CH (2018) Extraction optimization, purification, antioxidant activity, and preliminary structural characterization of crude polysaccharide from an Arctic Chlorella sp. Polymers 10:E292
Moreira JB, Vaz BD, Cardias BB, Cruz CG, Almeida AC, Costa JA, Morais MG (2022) Microalgae polysaccharides: an alternative source for food production and sustainable agriculture. Polysaccharides 3(2):441–457
Gómez-Zorita S, Trepiana J, González-Arceo M, Aguirre L, Milton-Laskibar I, González M, Eseberri I, Fernández-Quintela A, Portillo MP (2019) Anti-obesity effects of microalgae. Int J Mol Sci 21:41
Medeiros VPB, Souza EL, Albuquerque TMR, Sassi CFC, Lima MS, Sivieri K, Pimentel TC, Magnani M (2021) Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res 60:102547
Heo M-G, Choung S-Y (2018) Anti-obesity effects of Spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and SIRT1. Food Funct 9:4906–4915
Mishra N, Gupta E, Singh P, Prasad R (2021) Application of microalgae metabolites in food and pharmaceutical industry. In: Preparation of phytopharmaceuticals for the management of disorders. pp 391–408
Ferreira AF, Ferreira A, Dias APS et al (2020) Pyrolysis of Scenedesmus obliquus biomass following the treatment of different wastewaters. Bioenergy Res 13:896–906. https://doi.org/10.1007/s12155-020-10102-1
Khavari F, Saidijam M, Taheri M, Nouri F (2021) Microalgae: therapeutic potentials and applications. Mol Biol Rep 48(5):4757–4765
Kumar D, Kaštánek P, Adhikary SP (2018) Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr Sci 115(2):234–241
Hamed I, Jakobsen AN, Lerfall J (2022) Sustainable edible packaging systems based on active compounds from food processing byproducts: a review. Compr Rev Food Sci Food Saf 21:198–226
Shah MR, Lutzu GA, Alam A, Sarker P, Kabir Chowdhury MA, Parsaeimehr A, Liang Y, Daroch M (2018) Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol 30:197–213
Barboríková J, Šutovská M, Kazimierová I, Jošková M, Fraňová S, Kopecký J, Capek P (2019) Extracellular polysaccharide produced by Chlorella vulgaris—chemical characterization and anti-asthmatic profile. Int J Biol Macromol 135:1–1
Saadaoui I, Rasheed R, Aguilar A et al (2021) Microalgal-based feed: promising alternative feedstocks for livestock and poultry production. J Anim Sci Biotechnol 12:76. https://doi.org/10.1186/s40104-021-00593
Alvarez AL, Weyers SL, Goemann HM, Peyton BM, Gardner RD (2021) Microalgae, soil and plants: a critical review of microalgae as renewable resources for agriculture. Algal Res 54:102200
Renuka N, Guldhe A, Prasanna R, Singh P, Bux F (2018) Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnol Adv 36:1255–1273
Jochum M, Moncayo LP, Jo Y (2018) Microalgal cultivation for biofertilization in rice plants using a vertical semi-closed airlift photobioreactor. PLoS ONE 13:e0203456
Rachidi F, Benhima R, Sbabou L, El Arroussi H (2020) Microalgae polysaccharides bio-stimulating effect on tomato plants: growth and metabolic distribution. Biotechnol Rep 25:e00426
Righini H, Baraldi E, García Fernández Y, Martel Quintana A, Roberti R (2019) Different antifungal activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Mar Drugs 17:299
Stirk WA, Staden J (2020) Potential of phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol Adv 44:107612
Bhalamurugan GL, Valerie O, Mark L (2018) Valuable bioproducts obtained from microalgal biomass and their commercial applications: a review. Environ Eng Res 23:229–241
Martínez-Ruiz M, Martínez-González CA, Kim D-H, Santiesteban-Romero B, Reyes-Pardo H, Villaseñor-Zepeda KR, Meléndez-Sánchez ER et al (2022) Microalgae bioactive compounds to topical applications products—a review. Molecules 27(11):3512
Zhuang D, He N, Khoo KS, Ng EP, Chew KW, Ling TC (2022) Application progress of bioactive compounds in microalgae on pharmaceutical and cosmetics. Chemosphere 291:132932
Dixon C, Wilken LR (2018) Green microalgae biomolecule separations and recovery. Bioresour Bioprocess 5:14
Yarkent Ç, Gürlek C, Oncel SS (2020) Potential of microalgal compounds in trending natural cosmetics: a review. Sustain Chem Pharm 17:100304
Orejuela-Escobar L, Gualle A, Ochoa-Herrera V, Philippidis GP (2021) Prospects of microalgae for biomaterial production and environmental applications at biorefineries. Sustainability 13(6):3063
Flores-Contreras EA, Araújo RG, Rodríguez-Aguayo AA, Guzmán-Román M, García-Venegas JC, Nájera-Martínez EF, Sosa-Hernández JE, Iqbal HMN, Melchor-Martínez EM, Parra-Saldivar R (2023) Polysaccharides from the Sargassum and brown algae genus: extraction, purification, and their potential therapeutic applications. Plants 12(13):2445
Usov AI, Zelinsky ND (2013) 2—chemical structures of algal polysaccharides. In: Domínguez H (ed) Functional ingredients from algae for foods and nutraceuticals. Woodhead Publishing, Sawston, pp 23–86
Fernandes PAR, Coimbra MA (2023) The antioxidant activity of polysaccharides: a structure-function relationship overview. Carbohydr Polym 314:120965
Xu S-Y, Huang X, Cheong K-L (2017) Recent advances in marine algae polysaccharides: isolation, structure, and activities. Mar Drugs 15(12):388
Ibrahim TN, Feisal NA, Kamaludin NH, Cheah WY, How V, Bhatnagar A, Ma Z, Show PL (2023) Biological active metabolites from microalgae for healthcare and pharmaceutical industries: a comprehensive review. Bioresour Technol 372:128661
Pradhan B, Patra S, Dash SR, Nayak R, Behera C, Jena M (2021) Evaluation of the anti-bacterial activity of methanolic extract of Chlorella vulgaris Beyerinck [Beijerinck] with special reference to antioxidant modulation. Future J Pharm Sci 7(1):1–1
Anvar AA, Nowruzi B (2021) Bioactive properties of spirulina: a review. Microb Bioact 4:134–142
Vishwakarma J, Parmar V, Vavilala SL (2019) Nitrate stress-induced bioactive sulfated polysaccharides from Chlamydomonas reinhardtii. Biomed Res J 6(1):7
Wan X, Li X, Liu D, Gao X, Chen Y, Chen Z, Fu C, Lin L, Liu B, Zhao C (2021) Physicochemical characterization and antioxidant effects of green microalga Chlorella pyrenoidosa polysaccharide by regulation of microRNAs and gut microbiota in Caenorhabditis elegans. Int J Biol Macromol 168:152–162
Armaini A, Hajir S, Rilda Y (2022) Evaluation of lipid profile and liver function after administration of Scenedesmus dimorphus in obese mice. Jurnal Riset Kimia 13(2):216–225
Das A, Bai C-H, Chang J-S, Huang Y-L, Wang F-F, Chen Y-C, Chao JC-J (2023) Associations of dietary patterns and vitamin D levels with iron status in pregnant women: a cross-sectional study in Taiwan. Nutrients 15:1805
Cai B, Zhao X, Luo L, Wan P, Chen H, Pan J (2022) Structural characterization, and in vitro immunostimulatory and antitumor activity of an acid polysaccharide from Spirulina platensis. Int J Biol Macromol 196:46–53
Hyrslova I, Krausova G, Smolova J, Stankova B, Branyik T, Malinska H, Huttl M, Kana A, Doskocil I, Curda L (2021) Prebiotic and immunomodulatory properties of the microalga Chlorella vulgaris and its synergistic triglyceride-lowering effect with bifidobacteria. Fermentation 7(3):125
Zhang J, Liu L, Ren Y, Chen F (2019) Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int J Biol Macromol 128:761–767. https://doi.org/10.1016/j.ijbiomac.2019.02.009
Qiu Y, Gao X, Chen R, Lu S, Wan X, Farag MA, Zhao C (2022) Metabolomics and biochemical insights on the regulation of aging-related diabetes by a low-molecular-weight polysaccharide from green microalga Chlorella pyrenoidosa. Food Chem X 14:100316
Acknowledgements
Support of the central instrumental lab facility at Lovely Professional University, Phagwara, Punjab is gratefully acknowledged.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
SS: writing—original draft, data curation, formal analysis, conceptualization; AB: resource, software, writing—original draft—review & editing; MS: software, writing—original draft—review & editing; PC: supervision, conceptualization, resource, validation, writing—original draft—review & editing, project administration; KS: software, conceptualization, visualization, supervision; validation, writing—original draft—review & editing, project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selvaraj, S., Bains, A., Sharma, M. et al. Freshwater Edible Algae Polysaccharides: A Recent Overview of Novel Extraction Technologies, Characterization, and Future Food Applications. J Polym Environ (2023). https://doi.org/10.1007/s10924-023-03049-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-023-03049-9