Abstract
The present work investigated the impact of ultrafiltration (UF) and nanofiltration (NF) membranes on the recovery and fractionation of polyphenolic compounds and polysaccharides from Sangiovese and Cabernet Sauvignon wine lees. A laboratory-made flat-sheet membrane in cellulose acetate (CA400-38) was used in the UF treatment of Sangiovese wine lees; three laboratory-made flat-sheet membranes in cellulose acetate (CA316, CA316-70, CA400-22) and a polyamide commercial membrane (NF90) were used in the NF treatment of Cabernet Sauvignon wine lees. All membranes were characterized in terms of hydraulic permeability and rejection toward references solutes; the performances of the membranes were measured in terms of productivity, fouling index, cleaning efficiency and retention toward target compounds.
Experimental results indicated that all UF and NF membranes were effective in separating target compounds rejecting more than 92% of polysaccharides with polyphenols preferentially permeating through the membrane. The UF membrane rejected more than 40% of total polyphenols; rejections toward non-flavonoids and flavonoids were less than 25% and 12.5%, respectively.
The laboratory-made NF membranes exhibited higher permeate flux values (of the order of 11–12 L/m2h) in comparison with the commercial NF membrane, despite the observed differences in the retention of specific solutes. Among the prepared membranes the CA316 showed a total rejection toward most part of non-flavonoids and flavonoids.
The experimental results support the use of UF and NF processes in a sequential design to fractionate and refine phenolic compounds from winery sludge for the production of concentrated fractions with high antioxidant activities.
Similar content being viewed by others
Introduction
Grape cultivation is one of the most extensive crops in the world with an annual production of 92 million tons. The commercialization of the grape ranges from raw consumption to the production of juices, wines, jams, raising, vinegar, jelly, seed extract and seed oil; however, 80% of the whole grape production is destined to winemaking (Zhu et al., 2015; FAOSTAT-FAO, 2020).
At the primary production level vine shoots from pruning and cuttings are produced; then, stems and white grape pomaces are separated at the first stages of vinification, followed by red grape pomaces and wine lees following the fermentation (Grainger & Tattersall, 2007). The amount of waste generated by the winemaking process can vary due to different factors such as grape cultivation technique, pressing process and different steps of fermentation (Dwyer et al., 2014).
Solid residues regarding wine production correspond to approximately 30% of the grapes used, which is represented in millions of tons of residues (Toscano et al., 2013). Wastes generated during the winemaking process may have a strong environmental impact; in particular, large quantities of wastes are produced in a short period, such as during harvesting from August to October, which if discharged in landfill can be harmful for the environment. Indeed, the presence of phenolic compounds in these wastes affects germination and decreases the soil pH, thus determining an increase of their resistance to biological degradation (Inderjit, 1997). Other environmental problems are bad odors, attraction of undesirable pests, contamination of surface water and depletion of oxygen in the ground and groundwater (Arvanitoyannis et al., 2006; Chand et al., 2009).
For this reason, the winemaking industry is geared toward innovative and sustainable waste management systems, with an increasing demand for recycling or valorization strategies (Dwyer et al., 2014).
The most recent challenges concern the exploitation of wine industry wastes for obtaining bioactive compounds (mainly polyphenols) to be used as dietary supplements or natural antioxidants for several technological purposes; the published studies mainly focus on the recovery of bioactive polyphenols from solid wastes (i.e., wine pomaces) and liquid waste streams (Arvanitoyannis et al., 2006; Díaz et al., 2012; Moro et al., 2021). In this scenario the winemaking lees, traditionally exploited for the recovery of organic acids, are gaining increasing interest in relation to their polyphenolic content (De Iseppi et al., 2020).
Wine lees are generated following the fermentation process and constitute a large fraction of the solid wastes from winemaking. Wine lees are defined by the Council Regulation (n. 337/79) as the residue formed at the bottom of recipients containing wine, after fermentation, during storage or after authorized treatments, as well as the residue obtained following filtration or centrifugation of this product (Pérez-Serradilla & Luque de Castro, 2011). Their composition depends on the agronomic characteristics of grapes and their origin; on average, they represent 2 − 6% of the total volume of wine produced and consist of ethanol, tartaric acid, yeast cells and phenolic compounds (Pérez-Bibbins et al., 2015; Dimou et al., 2015; Delgado et al., 2015). It follows that wine lees are essentially rich in valuable compounds with nutritional and technological properties, offering several opportunities for their valorization.
Polyphenolic compounds are recognized for their nutraceutical properties, among others; their recovery from food and agricultural wastes is gaining an increasing interest in recent times, hypothesizing their re-usage as natural antioxidants in sustainable food processing chains (Troilo et al., 2021).
Nowadays, phenolic compounds are mainly obtained from their vegetal sources by solvent (ethanol, methanol, acetonitrile) extraction, which sets several limitations for their usage in the food industry, urging the importance of studies aimed at finding environmentally and economically sustainable alternatives (Amyrgialaki et al., 2014; Singh et al., 2014).
Membrane separation technology—and particularly pressure-driven membrane operations–has a prominent role in the scenario of emerging technologies, due to its versatility and multiple advantages: absence of phase transition, high separation efficiency, mild operating conditions and easy scaling up when compared with conventional and alternative extraction methods (Castro-Muñoz et al., 2017). It is recognized as a standard tool in the food industry (Mohammad et al., 2012) with well-established applications in fruit and beverage (Ambrosi et al., 2014; Gulec et al., 2017; Mondal et al., 2014), dairy (Pouliot, 2008), fish (Chabeaud et al., 2009) and poultry (Fatima et al., 2021) industries. In addition, pressure-driven membrane operations have gained a great interest in the last years for the recovery and purification of phenolic compounds from food by-products including those generated in the wine industry (Giacobbo et al., 2015, 2017; Giacobbo, Bernardes, et al., 2013; Giacobbo, Oliveira, et al., 2013; Kontogiannopoulos et al., 2017; Muñoz et al., 2021; Tapia-Quirós et al., 2022; Zagklis & Paraskeva, 2015). In this contest, this work was aimed at evaluating the potential of ultrafiltration (UF) and nanofiltration (NF) laboratory-made flat-sheet membranes in the fractionation of polyphenolic compounds and polysaccharides from Sangiovese and Cabernet Sauvignon wine lees. The membrane performance was measured in terms of productivity, fouling index, cleaning efficiency and retention toward target compounds.
The experimental results support the use of the membrane technologies to obtain enriched streams of antioxidant and polysaccharide compounds, which can be directed to the enological employment as adjuvant as well as to the creation of innovative products free of chemical additives of interest for pharmaceutical, cosmetic and food applications.
Materials and Methods
Feed Solution for the UF and NF Process
The Sangiovese wine lees for the UF process and Cabernet Sauvignon wine lees for the NF process were kindly provided by Cantina di Terre Naldi (Faenza, Emilia-Romagna, Italy). The wine lees were obtained by racking after fermentation of the grape musts and stored at -20 °C before use.
Membranes: Preparation and Characterization
UF Membranes
A cellulose acetate laboratory-made membrane, coded as CA400-38, was used for the UF experiments with Sangiovese wine lees. The UF membranes were prepared according to the phase inversion method as reported by Kunst and Sourirajan (1974). The casting solution composition and the casting conditions used to prepare the CA400-38 membrane are shown in Table 1. The cellulose acetate and the solvent system acetone/formamide were kept under mechanical stirring for 24 h, to obtain a homogeneous solution. This solution was casted on a clean glass plate with a casting knife with the gate height of 250 μm. The plate, after an evaporation time of 30 s, was immersed in an ice/water coagulation bath to allow the membrane formation. The cellulose acetate, acetone and formamide were supplied by Merck (Hohenbrunn, Germany).
The membrane was characterized in terms of water permeability and apparent rejection coefficients to the reference solutes polyethylene glycol of 10, 20 and 35 kDa and Dextran 40 kDa in aqueous solutions with a solute concentration of 600 ppm. The water permeability of the membrane was obtained by the linear regression of the pure water permeation as a function of transmembrane pressure (TMP) (0.25 – 2.25 bar).
NF Membranes
Three NF laboratory-made cellulose acetate membranes (CA316, CA316-70 and CA400-22) were prepared according to the phase inversion method reported by Kunst and Sourirajan (1974). Membrane casting solutions and casting conditions are reported in Table 2. Although the casting solution composition of the CA316 and CA316-70 is different from the CA400-22 and CA400-38 due to the introduction of the magnesium perchlorate in the substitution of the acetone/formamide solvent system, the casting solution preparation details follow the same procedure . The CA316-70 membrane is subjected to a post-formation annealing treatment in the conditions described in Table 2.
A polyamide commercial membrane, NF90 (DowFilmtec, USA), in flat-sheet configuration was used for comparison purpose in the NF experiments.
All NF membranes were characterized by hydraulic permeability and apparent rejection coefficients to the reference solutes D-( +)-glucose, raffinose, saccharose, polyethylene glycol of 1 kDa, sodium chloride, sodium sulfate and ethanol in aqueous solutions with a solute concentration of 600 ppm. The water permeability of the membranes was obtained by linear regression of the pure water permeation flux as a function of TMP (5, 10, 15 and 20 bar).
Experimental Set-up and Procedures
NF experiments were performed by using three laboratory-made cellulose acetate membranes prepared according to the phase inversion method reported by Kunst and Sourirajan (1974) and one commercial membrane (NF90) supplied by DowFilmtec (USA). Membrane-casting solutions and film-casting conditions are reported in Table 2. Cellulose acetate, acetone, formamide and magnesium perchlorate were of analyte grade (Merck Millipore, Darmstadt, Germany). A polyamide commercial membrane in flat-sheet configuration with a molecular weight cutoff (MWCO) of 200–400 Da (NF90, DowFilmtec, USA) was also used for NF experiments. All membranes were characterized by water permeability and reference rejection coefficients which include refined ( +)-glucose, polyethylene glycol (PEG 1,000 Da), sodium chloride, sodium sulfate and ethanol as the reference molecules using feed solutions with a solute concentration of 600 ppm. The water permeability of the membranes was obtained by linear regression of the water flux against the TMP.
Ultrafiltration
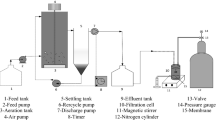
UF experiments were carried out by using a laboratory-scale filtration unit, P18-CELFA, that integrates a cylindrical jacketed feed tank with a capacity of 500 mL constructed from stainless steel, with a membrane filtration crossflow cell of an effective membrane surface area of 26.4 cm2 (Botelho et al., 2022). The system includes a feed pump, a pressure gauge, a thermometer placed inside the feed tank and a pressure-regulating valve. The adjustment of operating pressure and feed flowrate was done by simultaneously pump rotation control through a frequency inverter and the pressure-regulating valve. The operating temperature was controlled by circulating tap water through the tank jacket. Before permeation experiments, all membranes were compacted at 3 bar for 2 h to avoid pressure effects on membrane structure.
Filtration experiments were performed according to the batch concentration configuration in which the permeate is continuously collected, while the retentate is recycled back to the feed reservoir. The UF unit was operated under a TMP of 2 bar, at a temperature of 25 ± 1 °C and 0.55 L/min feed flowrate. The experiments were conducted to obtain 1.62 of volume reduction factor (VRF), which is defined as the ratio between the initial feed volume and the volume of the resulting retentate. At the end of each experimental run, the membrane was washed with deionized water to restore the filtration capacity.
Nanofiltration
The NF permeation experiments were performed in a crossflow filtration set-up consisting of a feed tank, a pump, a flowmeter, two manometers, four flat plates cells with a membrane surface area of 13.2 cm2 and a pressure control valve. Before permeation experiments, all membranes were compacted at 35 bar for 2 h, to avoid pressure effects on membrane structure.
The permeation experiments of Cabernet Sauvignon wine lees were carried out in total recirculation mode at 20 bar, where the permeate and retentate streams were recirculated to the feed tank under selected temperature conditions (25 ± 1 °C). The feed tank temperature was controlled using a thermostated bath, and for each experiment, a volume of 5 L of the feed solution was used and a stabilization time of 30 min.
UF/NF Permeation Characterization
The following membrane parameters were measured in both UF and NF processes.
The permeate flux (Jp), expressed as L/m2h, was calculated by measuring the collected permeate volume in a fixed time using following Eq. (1):
where Vp is the volume of permeate collected during the time interval t and A is the membrane surface area.
The fouling index (FI) of the investigated membranes was determined by measuring the hydraulic permeability (Wp) before and after the filtration processes, according to following Eq. (2):
where Wp0 and Wp1 are the pure water permeability before and after the filtration processes, respectively.
After the treatment of both Sangiovese and Cabernet Sauvignon wine lees by means of the UF and NF system, the membranes were washed with deionized water for 30 min and their permeability with deionized water was measured. The cleaning efficiency (CE) was evaluated according to following Eq. (3):
where Wp2 is the water permeability after the cleaning process and Wp0 is the water permeability before the filtration processes with wine lees.
The rejection (R) of UF and NF membranes toward specific compounds was calculated according to following Eq. (4):
where Cp and Cf are the permeate and feed solute concentrations, respectively.
Pretreatment of Wine Lees
The lees from Sangiovese and Cabernet Sauvignon wine were previously centrifuged at 4000 RPM for 10 min prior to filtration experiments with UF and NF membranes.
Analytical Determinations of the Filtration Products
Feed (F), permeate (P) and retentate (R) samples obtained from UF and NF experiments were analyzed in terms of total polyphenols, proanthocyanidins content, antioxidant capacity, mannoproteins, sugars (glucose and fructose), pH and turbidity.
Total polyphenols were quantified by means of the Folin-Ciocalteu method (TP-FC) and expressed as milligrams of gallic acid equivalent per liter of solution, mg GAE/L (Ribéreau-Gayon et al., 1976). The reaction was performed by mixing a 0.250 mL sample aliquot, 0.5 mL of Folin-Ciocalteu reagent (diluted 1:4) and 1.75 mL of a 15% sodium carbonate solution. The reaction mixture was incubated at room temperature for 1 h; then, the absorbance was read at 725 nm by using a UV–Vis spectrophotometer (UV-1700 Shimadzu, Shimadzu Scientific Instruments, Kyoto, Japan).
The quantification of proanthocyanidins was carried out by means of a spectrophotometric assay with the 4-dimethyl-amino cinnamaldehyde reagent (DMAC) and expressed as ( +)-catechin equivalents (mg CE/L) according to the literature (Wang et al., 2016) and using a UV–Vis spectrophotometer (UV-1700 Shimadzu) at a wavelength of 640 nm.
The sugar content (d-glucose and d-fructose), expressed in mg/L, was measured through an enzymatic assay (Megazyme, Chicago, USA) by using an Agilent Cary60 UV–Vis spectrophotometer (Agilent, Santa Clara, CA, USA).
The pH value was measured with a HANNA 209 pH meter (Merck, Germany) calibrated with buffer solutions at pH 4.0 and 7.0.
Turbidity was measured by using a compact Aqualytic® infrared turbidity meter (AL250T-IR, Germany) with a measurement range of 0.01 to 1110 nephelometric turbidity units (NTU) and a detection limit of 0.01 NTU.
The antioxidant capacity was measured according to the 2,2’-azino-bis-(-3-ethylbenzothiazoline-6-sulfonic acid) radical colorimetric assay (ABTS•+) as reported by Re et al. (1999) using an Agilent Cary60 UV–Vis spectrophotometer (Agilent, Santa Clara, CA, USA).
The antioxidant capacity (AC) was calculated as the percentage of inhibition of absorbance according to following Eq. (5):
where Abs0 is the absorbance value of the reagent blank at the beginning of the experiment (time zero) and Abs1 is the absorbance following sample addition and incubation. Results have been reported as percentage of the radical scavenging of the sample.
Total polysaccharides were measured using the colorimetric method developed by Segarra et al. (1995), using an Agilent Cary60 UV–Vis spectrophotometer (Agilent, Santa Clara, CA, USA).
The determination of mannoproteins was carried out isolating the polysaccharides through the HCl-ethanol solution, and subsequently, they were centrifuged according to Guadalupe et al. (2007). Subsequently, mannoproteins were separated based on the method described in the Resolution OENO 26/2004 (OIV, 2004). Finally, mannoproteins were quantified by means of the enzymatic assay (Megazyme, Chicago, USA).
HPLC quantitative analyses of phenolic fraction and anthocyanins monomers were performed on feed, permeate and retentate samples after UF and NF of wine lees, respectively. Feed, permeate and retentate samples were previously filtered using a capsule nylon filter of 0.45 µm to remove suspends particles. HPLC analyses were performed by using an Agilent 1290 Infinity LC System with ultraviolet–visible (UV–Vis) Diode Array Detection (DAD) (Agilent, Palo Alto, CA, USA) and according to the methods described by Ivanova-Petropulos et al. (2015). Results were expressed by using calibration curves of the reference standard polyphenolic compounds. Standard polyphenolic monomers and malvidin-3-O-glucosyde used for HPLC calibration were purchased from Extrasynthese (Genay, France).
Statistical Analysis
Data are presented as the mean values ± standard deviation (SD) obtained from three replicates. The one-way analysis of variance (ANOVA A; significance p ≤ 0.05) and the Tukey’s HSD post hoc test were performed using Minitab® 17.1.0 (Minitab, Ltd. UK) statistical software.
Results and Discussion
UF of Sangiovese Wine Lees
Membrane Characterization
The rejection of the UF membrane toward references solutes (PEG and dextran of different molecular weight) resulted higher than 91%. The highest rejection was observed for the PEG 35 kDa. The curve fitting of plot log (f/(1-f)) as a function of the solute molecular weight was intersected by the 99% rejection line and yielded a molecular weight cutoff of 35 kDa (Fig. 1).
The permeate flux of the pure water was measured at different transmembrane pressures from 0.25 to 2.25 bar. The permeate flux was plotted as a function of TMP, and the membrane hydraulic permeability was obtained as the slope of the straight line from that plot (see Figure S1 in supplementary data).
Physicochemical Composition of Sangiovese Red Wine Lees
Table 3 shows the physicochemical composition of the Sangiovese red wine lees used as feed of the filtration experiments. The pH value detected for this sample (pH = 3.7) is at the limit of the acidity values normally detected in wine lees (3.8 < pH < 6.8) (Bustamante et al., 2008). However, the pH value is strictly correlated with the wine production process. It was found that the total polyphenols resulted in 655.4 mg GAE/L. Typically, the range of polyphenol content in wine lees from different varieties is about 400 – 1000 mg GAE/L (Giacobbo et al., 2015; Lužar et al., 2016).
The wine lees contained 0.358 of polysaccharides expressed in g glucose/L. A different result was obtained by Giacobbo, Bernardes, et al. (2013) reporting 0.0498 g glucose/L in the second racking from Syrah wine lees. The higher content of polysaccharides measured in our sample could be attributed to the fact that the racking operation was carried out only once.
The mannoproteins concentration in the Sangiovese wine lees was 0.39 g/L. The production and release of mannoproteins during wine fermentation depends on the yeast strain and will consequently affect the concentration of mannoproteins remaining in the wine lees (Vidal et al., 2003).
The Sangiovese wine lees contained 0.712 g/L of total sugars, including glucose and fructose. Galanakis et al. (2013) obtained significantly different results reporting total sugar concentrations of 1.065 g/L and 3.91 g/L in diluted and concentrated hydro-ethanol extracts of wine lees, respectively. However, the concentration of total sugars can vary in wine lees because it depends on the vinification process adopted and, therefore, on the residual unfermented sugars.
Filtration Experiments
Figure 2 shows the time evolution of the permeate flux and VRF in the treatment of Sangiovese wine lees with the CA400-38 membrane in the selected operating conditions. The initial permeate flux was approximately 7.82 L/m2h and gradually decreased until reaching a steady-state value of approximately 5.25 L/m2h. In particular, a rapid decrease of the permeate flux was observed in the first 245 min of the process with a reduction of 21.23% with respect to the initial permeate flux. Then the permeate flux decreased gradually up to reach a steady-state value. This behavior can be attributed to different phenomena including membrane fouling, concentration polarization and an increase in the concentration of retained solutes in the feed tank. Indeed, as the feed concentration increases, the concentration polarization becomes more severe. Accumulation of solutes occurs toward the membrane surface, leading to the formation of a gelatinous-type layer which is responsible for an additional resistance to the permeate flux in addition to that of the membrane (Cassano, Marchio, et al., 2007). A similar behavior was observed by Castro-Muñoz et al. (2017) in the UF treatment of xoconostle juice (Opuntia joconostle) with polysulfone hollow fiber membranes. Authors observed a rapid decrease of the permeate flux in the first step of the process followed by a slow decline up to reach a steady-state flux.
According to the final VRF of the process (1.62), 192 mL of permeate and 308 mL of retentate were collected, respectively. This means, 38.4% of the initial Sangiovese wine lees was recovered as clarified solution.
Fouling Index and Cleaning Efficiency
The hydraulic permeability of the UF membrane was close to 23.45 L/m2hbar; it decreased up to 9.80 L/m2hbar after the UF process (see Figure S1 in supplementary data). According to these data, the fouling index and the cleaning efficiency of the membrane were of 41.8% and 42.9%, respectively. Similar values were reported by Cassano, Donato, et al. (2007) in the clarification of kiwifruit juice with polyvinylidenefluoride membranes of 15 kDa in tubular configuration. Membrane fouling is influenced by different factors including the physicochemical composition of the solution, polarization concentration phenomena, adsorption effects and electrostatic interactions, characteristics of the membrane (MWCO, configuration, membrane material, hydrophobicity, porosity and surface charge) and operating conditions (TMP, feed flowrate and temperature) (Boussu et al., 2006; Cassano, Donato, et al., 2007).
Analyses of Membrane Selectivity
According to the composition of feed, permeate and retentate samples from the UF treatment of Sangiovese wine lees (Table 3) the major effect of the filtration process was observed in the optical properties of the permeate solution. Indeed, the turbidity of the original wine lees, due to residual yeasts, lactic bacteria from alcoholic and malolactic fermentation and residual cells (Vernhet & Moutounet, 2002) was reduced by 98.5% (from 1,000 to 1.5 NTU) following the UF treatment. On the other hand, the pH was not significantly modified along the filtration process with respect to the value measured in the feed solution. According to the results reported in Table 3, most part of polysaccharides were recovered in the retentate stream.
Figure 3 displays the rejection of the UF membrane toward the different compounds of the Sangiovese wine lees. In particular, the rejection of the UF membrane toward polysaccharides resulted of about 92%. A similar value was reported by Giacobbo et al. (2015) in the treatment of Merlot wine lees with microfiltration membranes (around 95% of rejection). In the same work, the authors reported a retention of 74.7% toward the polyphenolic content using polyimide hollow fiber membrane with 0.4 µm pore size. Similarly, Galanakis et al. (2013) reported a rejection of about 90% for polysaccharides from wine lees using a polyethersulfone UF membrane with a MWCO of 7,600 Da. By referring to the phenolic compounds the observed rejection of the UF membrane was of about 41%. A similar result was reported by Streit et al. (2009) in the treatment of leather effluents containing salts and organic compounds. The authors used a fluoropolymer composite UF membrane with a MWCO of 1,000 Da which exhibited 45% rejection toward tannins. Therefore, the set of results indicate a preferential permeation of phenolic compounds over polysaccharides through the UF membrane.
Table 4 displays the HPLC quantitative analyses of phenolic fraction and anthocyanins monomers in the feed, permeate and retentate samples of the UF process. The analyses revealed a small increase in the concentration of phenolic compounds and anthocyanin monomers on the retentate side, even so the compositions of the permeate and retentate fractions were similar. A similar behavior was observed by Conidi et al. (2011) for flavonoids compounds of bergamot juice treated with polysulfone hollow fiber membranes with a nominal MWCO of 1 kDa.
Despite the high fouling index of 41.8%, the retention of some phenolic fractions such as ( +)—catechin, (-) – epigallocatechin, quercetin aglycone and malvidin-3-O-glucoside was lower than 5%, while other phenolic compounds like vanillic acid, gallic acid, syringic acid, fertaric acid, coutaric acid, chlorogenic acid, caffeic acid and other anthocyanin monomers, including cyanidin-3-O-glucoside, petunidin-3-O-glucoside and peonidin-3-O-glucoside, were rejected in the range from 5 to 10%. This phenomenon can be attributed to the large pore diameter of the UF membrane (35000 Da) allowing the diffusion of compounds with low molecular weight. It should be noted that these compounds have a molecular weight lower than 1,000 Da (Garcia et al., 1999; He et al., 2016). Conidi et al. (2017) reported a retention of 6.9% of cyanidin 3-O-glucoside in the clarification of pomegranate juice with a composite fluoropolymer flat sheet UF membrane having a MWCO of 1,000 Da.
The UF membrane presented a high rejection toward mannoproteins (rejection coefficient of 100%) and a permeation preference toward flavonoids and non-flavonoids, including anthocyanin monomers (rejection less than 25% for all analyzed compounds). In addition, it exhibited a high rejection coefficient toward polysaccharides. This behavior makes possible the fractionation of low molecular weight polyphenols and polysaccharides by UF, since polyphenols preferably permeate through the membrane, while mannoproteins and polysaccharides are mainly retained.
The optimal polysaccharides/polyphenols fractionation was achieved with a fouling index of 41.8%. This value can be lowered trough the minimization of the concentration polarization by the increasing of the feed circulation velocity.
NF of Cabernet Sauvignon Wine Lees
Membrane Characterization
In Table 5 the rejection of both laboratory made and commercial NF membranes toward a set of solutes, salts and ethanol is reported. According to the experimental results, the NF90 membrane presented the highest rejection for all investigated compounds, followed by the CA316-70, CA316 and CA400-22 membrane. In particular, the NF90 and CA316-70 membranes showed rejections of 99% and 97% toward sodium sulfate, respectively, and 95% and 77% toward sodium chloride, respectively. On the other hand, the membranes CA400-22 and CA316 presented lower rejections to sodium sulfate (47% and 86%, respectively) and sodium chloride (10% and 27%, respectively) in comparison with the other membranes.
The water permeability data (see Figure S2-S5 in supplementary data) agreed with the typical values of NF membranes. The highest water permeability was measured for the CA400-22 membrane (8.34 L/m2bar); on the other hand, the NF90 membrane showed the lowest value (3.75 L/m2bar).
Physicochemical Composition of Cabernet Sauvignon Wine Lees
Table 6 shows the physicochemical composition of the Cabernet Sauvignon wine lees used in the NF experiments. The pH value measured for the feed solution was 3.8, resembling previous values from the literature (Giacobbo, Oliveira, et al., 2013). The total polyphenolic content in the wine lees was 384.1 mg GAE/L. Higher values (about 476 mg GAE/L) were reported by Galanakis et al. (2013) for the aqueous extract of Maratheftiko wine lees.
The wine lees contained 1.67 g/L and 0.05 g/L of glucose and fructose, respectively. The glucose content in the wine lees was higher than fructose; however, the content of these sugars resulted lower than those reported by Arboleda Mejia et al. (2019) for mixed red wine lees (0.58 g/L and 0.03 g/L, respectively).
The content of proanthocyanidins in the wine lees was 12.2 mg CE/L. Arboleda Mejia et al. (2019) reported a proanthocyanidin content of 6.9 mg CE/L for mixed red wine lees.
The radical scavenging activity measured by the ABTS assay was of 62.2% ± 1.9; it is positively correlated with the concentration of polyphenolic compounds (Floegel et al., 2011).
Membrane Productivity
The average permeate fluxes (Jp) measured in the treatment of the Cabernet Sauvignon red wine lees at an operating pressure of 20 bar resulted of about 12.46 L/m2h for the CA316-70 membrane, 12.28 L/m2h for the CA316 membrane and 11.25 L/m2h for the CA400-22 membrane. On the other hand, the NF90 showed a lower permeate flux with a value of 3.72 L/m2h. It is worth noting that these membranes presented similar productivity values despite the observed differences in the retention of specific solutes. On the other hand, a strong correlation between the cutoff these membranes and the permeate flux measured in the treatment of red grape pomace extract was observed in a previous work (Arboleda Mejia et al., 2020). For the NF90 membrane a good correlation between the rejection values of solutes and productivity was observed.
Fouling Index and Cleaning Efficiency
The fouling index for the investigated membranes was calculated based on the water permeability measured before and after the nanofiltration treatment of the Cabernet Sauvignon wine lees.
According to the results presented in Table 7, the NF90 membrane showed the highest fouling index with a value of 42.28%, followed by the CA400-22 membrane (23.84%), the CA316-70 membrane (9.57%) and finally the CA316 membrane (8.63%). The highest fouling index reported for the NF90 membrane could be explained due to the adsorption of organic compounds on the surface of the membrane through the formation of hydrogen bonds between the polymeric membrane and organic compounds. It is well known that the adsorption of polyphenolic compounds on the membrane surface could be promoted by hydrophobic interactions with the membrane material, which plays an important role in the retention of solutes, a high fouling index and a large decrease in the permeate flux (Arsuaga et al., 2010; Jönsson et al., 1995). The three laboratory-made cellulose acetate membranes showed a lower fouling index compared to the NF90 membrane. In addition, a total recovery of the hydraulic permeability was observed for the CA316-70 membrane after cleaning with distilled water. Cleaning efficiencies higher than 80% were measured for the CA316 and CA400-22 membranes. According to the highest fouling index, which was presented by the NF90 membrane, the cleaning efficiency for this membrane was the lowest with a recovery of water permeability of 58.02%. Different phenomena can explain the incomplete recovery of the hydraulic permeability of the membrane, such as irreversible fouling which is governed by the absorption effect of phenolic compounds on the surface of the membranes (Sotto et al., 2013).
Analyses of Membrane Selectivity
According to data reported in Table 6, minimal changes in pH values were noted in all permeate fractions in comparison with the feed solution. A minimal change in pH value was also reported by Giacobbo et al. (2015) in the microfiltration of the Merlot wine lees with a polyamide membrane; authors reported a change in pH in the permeate solution from 3.78 to 3.92.
Figure 4 shows the rejection of the NF membranes toward the analyzed compounds of Cabernet Sauvignon wine lees. All the membranes allowed a significant reduction of the wine lees turbidity, with rejection values of 97.7% for the NF90 membrane and 99.9% for all the laboratory-made membranes. Among the investigated membranes, the NF90 presented the highest rejection coefficient for total polyphenolic compounds and antioxidant capacity with values of 94 and 77%, respectively. This membrane allowed also a total removal of proanthocyanidins from the wine lees.
The CA400-22 membrane showed a rejection of proanthocyanidins close to 7% and a rejection coefficient of 66.5% to total polyphenols. The rejection coefficient for glucose and fructose was of about 40%. Rejections for phenolic compounds and sugars higher than 70% and 90%, respectively, were detected for the CA316 and CA316-70 membranes. Similar results were presented by Galanakis et al. (2013), reporting a rejection of 81% for polyphenolic compounds and 74% for total sugars in the UF treatment of Cypriot wine lees with a 100 kDa polysulfone membrane. Rejections higher than 80% for total phenols associated with low rejections for glucose and fructose have been reported recently by Mondal et al. (2021) in the filtration of pomegranate juice with polyamide membranes having a MWCO in the range of 1–3.5 kDa.
All the NF membranes presented a rejection higher than 96% for both polysaccharides and mannoproteins. This result is in accordance with the one reported by Giacobbo et al. (2017) which achieved a rejection of 99% for polysaccharides of Merlot wine lees using a nanofiltration membrane with a MWCO of 200–330 Da.
Table 8 shows the HPLC analyses of phenolic compounds and anthocyanins monomers in the feed and permeate streams of the NF process; the rejection coefficients for these compounds are reported in Table 9. All NF membranes presented a high rejection coefficient (above 70%) for two flavonols: myricetin and quercetin aglycone. This behavior can be attributed to their higher molecular weight (myricetin = 318 Da and quercetin aglycone = 302 Da) in comparison with the other flavonoids.
The CA316 membrane presented the highest rejection coefficients for all flavonoids (higher than 91%) and non-flavonoids (higher than 65%), while the CA400-22 membrane presented the lowest ones. Based on experimental results, the CA400-22 membrane seems to favor the separation of some species of hydroxycinnamic acids from anthocyanins having a low hydroxycinnamic acid rejection coefficient and a high rejection coefficient for anthocyanins. Likewise, a permeate stream rich in hydroxycinnamic acids with a low anthocyanin content can be obtained with this membrane.
This membrane presented also the largest gap between the rejection coefficients for polysaccharides and phenolic compounds highlighting his suitability for the fractionation of phenolic compounds and polysaccharides. On the other hand, the treatment of wine lees with the CA316 membrane provides a concentrated stream of polysaccharides and polyphenols. In this view a two-step process consisting of a pretreatment of the wine lees with the UF membrane followed by the concentration of the UF permeate with the CA316 membrane is another viable approach to fractionate polyphenols and polysaccharides from wine lees.
Conclusions
The current study suggests that the fractionation of phenolic compounds from polysaccharides in Sangiovese and Cabernet Sauvignon wine lees is possible by using UF and NF membranes.
Regarding the UF of Sangiovese wine lees, the results showed a high selectivity of a cellulose acetate laboratory-made membrane (CA400-38) toward polysaccharides (rejection of about 92%) and mannoproteins (rejection of 100%) and a high recovery of phenolic compounds (rejection of about 40%) in the permeate stream. In addition, this membrane exhibited low retention values for glucose and fructose (10.4% and 4.7%, respectively). Therefore, this membrane is a suitable candidate for phenolic/polysaccharides fractionation.
Laboratory-made NF membranes in cellulose acetate (CA316-70, CA316 and CA400-22) all presented high productivity in comparison with a commercial membrane. All these membranes presented a rejection higher than 96% toward polysaccharides. The permeate fraction obtained from the CA400-22 membrane presented the highest content of phenolic compounds and resulted depleted in both polysaccharides and mannoproteins. The CA316 membrane rejected most of polysaccharides and polyphenols: therefore, its combination with the UF membrane in a two-step process could be a viable approach to produce a concentrated solution with high antioxidant activity.
Code Availability
Not applicable.
Change history
20 July 2022
Missing Open Access funding information has been added in the Funding Note.
References
Ambrosi, A., Medeiros Cardozo, N. S., & Tessaro, I. C. (2014). Membrane separation processes for the beer industry: A review and state of the art. Food and Bioprocess Technology, 7, 921–936. https://doi.org/10.1007/s11947-014-1275-0
Arboleda Mejia, J. A., Parpinello, G. P., Versari, A., Conidi, C., & Cassano, A. (2019). Microwave-assisted extraction and membrane-based separation of biophenols from red wine lees. Food and Bioproducts Processing, 117, 74–83. https://doi.org/10.1016/j.fbp.2019.06.020
Arboleda Mejia, J. A., Ricci, A., Figueiredo, A. S., Versari, A., Cassano, A., Parpinello, G. P., & De Pinho, M. N. (2020). Recovery of phenolic compounds from red grape pomace extract through nanofiltration membranes. Foods, 9, 1–14. https://doi.org/10.3390/foods9111649
Amyrgialaki, E., Makris, D. P., Mauromoustakos, A., & Kefalas, P. (2014). Optimisation of the extraction of pomegranate (Punica granatum) husk phenolics using water/ethanol solvent systems and response surface methodology. Industrial Crops and Products, 59, 216–222. https://doi.org/10.1016/j.indcrop.2014.05.011
Arsuaga, J. M., López-Muñoz, M. J., & Sotto, A. (2010). Correlation between retention and adsorption of phenolic compounds in nanofiltration membranes. Desalination, 250(2), 829–832. https://doi.org/10.1016/j.desal.2008.11.051
Arvanitoyannis, I. S., Ladas, D., & Mavromatis, A. (2006). Potential uses and applications of treated wine waste: A review. International Journal of Food Science and Technology, 41(5), 475–487. https://doi.org/10.1111/j.1365-2621.2005.01111.x
Botelho, V. A., Mateus, M., Petrus, J. C. C., & de Pinho, M. N. (2022). Membrane Bioreactor for Simultaneous Synthesis and Fractionation of Oligosaccharides. Membranes, 12(2), 171. https://doi.org/10.3390/membranes12020171
Boussu, K., Van der Bruggen, B., Volodin, A., Van Haesendonck, C., Delcour, J. A., Van der Meeren, P., & Vandecasteele, C. (2006). Characterization of commercial nanofiltration membranes and comparison with self-made polyethersulfone membranes. Desalination, 191(1–3), 245–253. https://doi.org/10.1016/j.desal.2005.07.025
Bustamante, M. A., Moral, R., Paredes, C., Pérez-Espinosa, A., Moreno-Caselles, J., & Pérez-Murcia, M. D. (2008). Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Management, 28(2), 372–380. https://doi.org/10.1016/j.wasman.2007.01.013
Cassano, A., Marchio, M., & Drioli, E. (2007a). Clarification of blood orange juice by ultrafiltration: Analyses of operating parameters, membrane fouling and juice quality. Desalination, 212, 15–27. https://doi.org/10.1016/j.desal.2006.08.013
Cassano, A., Donato, L., & Drioli, E. (2007b). Ultrafiltration of kiwifruit juice: Operating parameters, juice quality and membrane fouling. Journal of Food Engineering, 79(2), 613–621. https://doi.org/10.1016/j.jfoodeng.2006.02.020
Castro-Muñoz, R., Fíla, V., Barragán-Huerta, B. E., Yáñez-Fernández, J., Piña-Rosas, J. A., & Arboleda-Mejía, J. (2017). Processing of Xoconostle fruit (Opuntia joconostle) juice for improving its commercialization using membrane filtration. Journal of Food Processing and Preservation, 42(1), e13394. https://doi.org/10.1111/jfpp.13394
Chabeaud, A., Vandanjon, L., Bourseau, P., Jaouen, P., ChaplainDerouiniot, M., & Guerard, F. (2009). Performances of ultrafiltration membranes for fractionating a fish protein hydrolysate: Application to the refining of bioactive peptidic fractions. Separation and Purification Technology, 66, 463–471. https://doi.org/10.1016/j.seppur.2009.02.012
Chand, R., Narimura, K., Kawakita, H., Ohto, K., Watari, T., & Inoue, K. (2009). Grape waste as a biosorbent for removing Cr(VI) from aqueous solution. Journal of Hazardous Materials, 163(1), 245–250. https://doi.org/10.1016/j.jhazmat.2008.06.084
Conidi, C., Cassano, A., & Drioli, E. (2011). A membrane-based study for the recovery of polyphenols from bergamot juice. Journal of Membrane Science, 375(1–2), 182–190. https://doi.org/10.1016/j.memsci.2011.03.035
Conidi, C., Cassano, A., Caiazzo, F., & Drioli, E. (2017). Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. Journal of Food Engineering, 195, 1–13. https://doi.org/10.1016/j.jfoodeng.2016.09.017
De Iseppi, A., Lomolino, G., Marangon, M., & Curioni, A. (2020). Current and future strategies for wine yeast lees valorization. Food Research International, 137, 109352. https://doi.org/10.1016/j.foodres.2020.109352
Delgado De La Torre, M. P., Priego-Capote, F., & Luque De Castro, M. D. (2015). Tentative identification of polar and mid-polar compounds in extracts from wine lees by liquid chromatography-tandem mass spectrometry in high-resolution mode. Journal of Mass Spectrometry, 50, 826–837. https://doi.org/10.1002/jms.3592
Díaz, B., Gomes, A., Freitas, M., Fernandes, E., Nogueira, D. R., González, J., Moure, A., Levoso, A., Vinardell, M. P., Mitjans, M., & Domínguez, H. (2012). Valuable polyphenolic antioxidants from wine vinasses. Food and Bioprocess Technology, 5, 2708–2716. https://doi.org/10.1007/s11947-011-0569-8
Dimou, C., Kopsahelis, N., Papadaki, A., Papanikolaou, S., Kookos, I. K., Mandala, I., & Koutinas, A. A. (2015). Wine lees valorization: Biorefinery development including production of a generic fermentation feedstock employed for poly(3-hydroxybutyrate) synthesis. Food Research International, 73, 81–87. https://doi.org/10.1016/j.foodres.2015.02.020
Dwyer, K., Hosseinian, F., & Rod, M. (2014). The market potential of grape waste alternatives. Journal of Food Research, 3(2), 91. https://doi.org/10.5539/jfr.v3n2p91
FAOSTAT-FAO. (2020). Statistical Database. Retrieved March 2020 from http://www.fao.org
Fatima, F., Du, H. B., & Kommalapati, R. R. (2021). Treatment of poultry slaughterhouse wastewater with membrane technologies: A review. Water, 13, 1905. https://doi.org/10.3390/w13141905
Floegel, A., Kim, D. O., Chung, S. J., Koo, S. I., & Chun, O. K. (2011). Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. Journal of Food Composition and Analysis, 24(7), 1043–1048. https://doi.org/10.1016/j.jfca.2011.01.008
Galanakis, C. M., Markouli, E., & Gekas, V. (2013). Recovery and fractionation of different phenolic classes from winery sludge using ultrafiltration. Separation and Purification Technology, 107, 245–251. https://doi.org/10.1016/j.seppur.2013.01.034
Garcia, A., Bonen, M., Vick-Ramírez, J., Sadaka, M., & Vuppu, A. (1999). Bioseparation Process Science. Blackwell.
Giacobbo, A., Bernardes, A. M., & de Pinho, M. N. (2013a). Nanofiltration for the recovery of low molecular weight polysaccharides and polyphenols from winery effluents. Separation Science and Technology, 48(17), 2524–2530. https://doi.org/10.1080/01496395.2013.809762
Giacobbo, A., Oliveira, M., Duarte, E. C. N. F., Mira, H. M. C., Bernardes, A. M., & de Pinho, M. N. (2013b). Ultrafiltration based process for the recovery of polysaccharides and polyphenols from winery effluents. Separation Science and Technology, 48(3), 438–444. https://doi.org/10.1080/01496395.2012.725793
Giacobbo, A., Do Prado, J. M., Meneguzzi, A., Bernardes, A. M., & De Pinho, M. N. (2015). Microfiltration for the recovery of polyphenols from winery effluents. Separation and Purification Technology, 143, 12–18. https://doi.org/10.1016/j.seppur.2015.01.019
Giacobbo, A., Bernardes, A. M., & de Pinho, M. N. (2017). Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Separation and Purification Technology, 173, 49–54. https://doi.org/10.1016/j.seppur.2016.09.007
Grainger, K., & Tattersall, H. (2007). Wine Production: Vine To Bottle. Vine To Bottle, Blackwell Publishing Ltd., Oxford.
Guadalupe, Z., Palacios, A., & Ayestarán, B. (2007). Maceration enzymes and mannoproteins: A possible strategy to increase colloidal stability and color extraction in red wines. Journal of Agricultural and Food Chemistry, 55(12), 4854–4862. https://doi.org/10.1021/jf063585a
Gulec, H. A., Bagci, P. O., & Bagci, U. (2017). Clarification of apple juice using polymeric ultrafiltration membranes: A comparative evaluation of membrane fouling and juice quality. Food and Bioprocess Technology, 10, 875–885. https://doi.org/10.1007/s11947-017-1871-x
He, B., Zhang, L. L., Yue, X. Y., Liang, J., Jiang, J., Gao, X. L., & Yue, P. X. (2016). Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chemistry, 204, 70–76. https://doi.org/10.1016/j.foodchem.2016.02.094
Inderjit, A. U. M. (1997). Effect of phenolic compounds on selected soil properties. Forest Ecology and Management, 92(1–3), 11–18. https://doi.org/10.1016/S0378-1127(96)03957-6
Ivanova-Petropulos, V., Ricci, A., Nedelkovski, D., Dimovska, V., Parpinello, G. P., & Versari, A. (2015). Targeted analysis of bioactive phenolic compounds and antioxidant activity of Macedonian red wines. Food Chemistry, 171, 412–420. https://doi.org/10.1016/j.foodchem.2014.09.014
Jönsson, C., & Jönsson, A. S. (1995). Influence of the membrane material on the adsorptive fouling of ultrafiltration membranes. Journal of Membrane Science, 108(1–2), 79–87. https://doi.org/10.1016/0376-7388(95)00144-X
Kontogiannopoulos, K. N., Patsios, S. I., Mitrouli, S. T., & Karabelas, A. J. (2017). Tartaric acid and polyphenols recovery from winery waste lees using membrane separation processes. Journal of Chemical Technology and Biotechnology, 92(12), 2934–2943. https://doi.org/10.1002/jctb.5313
Kunst, B., & Sourirajan, S. (1974). An approach to the development of cellulose acetate ultrafiltration membranes. Journal of Applied Polymer Science, 18(11), 3423–3434. https://doi.org/10.1002/app.1974.070181121
Lužar, J., Jug, T., Jamnik, P., & Košmerl, T. (2016). Comparison of total polyphenols content and antioxidant potential of wines from “Welschriesling” and “Sauvignon Blanc” varieties during ageing on fine lees. Acta Agriculturae Slovenica, 107(2), 473–482. https://doi.org/10.14720/aas.2016.107.2.18
Mohammad, A. W., Ng, C. Y., Lim, Y. P., & Ng, G. H. (2012). Ultrafiltration in food processing industry: Review on application, membrane fouling, and fouling control. Food and Bioprocess Technology, 5, 1143–1156. https://doi.org/10.1007/s11947-012-0806-9
Mondal, S., Cassano, A., & De, S. (2014). Modeling of gel layer-controlled fruit juice microfiltration in a radial cross flow cell. Food and Bioprocess Technology, 7, 355–370. https://doi.org/10.1007/s11947-013-1077-9
Mondal, S., Cassano, A., Conidi, C., & De, S. (2021). Quantification of selective transport of fructose and glucose during membrane filtration of pomegranate juice. Food and Bioprocess Technology, 14, 272–286. https://doi.org/10.1007/s11947-020-02558-y
Moro, K. I. B., Bender, A. B. B., da Silva, L. P., & Penna, N. G. (2021). Green extraction methods and microencapsulation technologies of phenolic compounds from grape pomace: A review. Food and Bioprocess Technology, 14, 1407–1431. https://doi.org/10.1007/s11947-021-02665-4
Muñoz, P., Pérez, K., Cassano, A., & Ruby-Figueroa, R. (2021). Recovery of anthocyanins and monosaccharides from grape marc extract by nanofiltration membranes. Molecules, 26(7), 1–12. https://doi.org/10.3390/molecules26072003
OIV. (2014). Resolution OENO 26/2004 ; OIV : Paris, France.
Pérez-Serradilla, J. A., & Luque de Castro, M. D. (2011). Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chemistry, 124(4), 1652–1659. https://doi.org/10.1016/j.foodchem.2010.07.046
Pérez-Bibbins, B., Torrado-Agrasar, A., Salgado, J. M., De Souza, R. P., & Domínguez, J. M. (2015). Potential of lees from wine, beer and cider manufacturing as a source of economic nutrients: An overview. Waste Management, 40, 72–81. https://doi.org/10.1016/j.wasman.2015.03.009
Pouliot, Y. (2008). Membrane processes in dairy technology-from a simple idea to worldwide panacea. International Dairy Journal, 18, 735–740. https://doi.org/10.1016/j.idairyj.2008.03.005
Ribéreau-Gayon, J., Peynaud, E., Supraud, S., & Ribéreau-Gayon, P. (1976). Sciences et techniques du vin: Analyse et contrôle des vins (p. 645). Dunod.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(98), 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Segarra, L., Lao, C., Lopez-Tamases, E., & de la Torre-Boronat, E. (1995). Spectrophotometric methods for the analysis of polysaccharide levels in winemaking products. American Journal of Enology and Viticulture, 46(4), 564–570.
Singh, M., Jha, A., Kumar, A., Hettiarachchy, N., Rai, A. K., & Sharma, D. (2014). Influence of the solvents on the extraction of major phenolic compounds (Punicalagin, ellagic acid and gallic acid) and their antioxidant activities in pomegranate aril. Journal of Food Science and Technology, 51(9), 2070–2077. https://doi.org/10.1007/s13197-014-1267-0
Sotto, A., Arsuaga, J. M., & Van der Bruggen, B. (2013). Sorption of phenolic compounds on NF/RO membrane surfaces: Influence on membrane performance. Desalination, 309, 64–73. https://doi.org/10.1016/j.desal.2012.09.023
Streit, K. F., Ferreira, J. Z., Bernardes, A. M., & De Pinho, M. N. (2009). Ultrafiltration/nanofiltration for the tertiary treatment of leather industry effluents. Environmental Science and Technology, 43(24), 9130–9135. https://doi.org/10.1021/es902105q
Tapia-Quirós, P., Montenegro-Landívar, M. F., Reig, M., Vecino, X., Cortina, J. L., Saurina, J., & Granados, M. (2022). Recovery of polyphenols from agri-food by-products: The olive oil and winery industries cases. Foods, 11(3), 1–26. https://doi.org/10.3390/foods11030362
Toscano, G., Riva, G., Duca, D., Pedretti, E. F., Corinaldesi, F., & Rossini, G. (2013). Analysis of the characteristics of the residues of the wine production chain finalized to their industrial and energy recovery. Biomass and Bioenergy, 55, 260–267. https://doi.org/10.1016/j.biombioe.2013.02.015
Troilo, M., Difonzo, G., Paradiso, V. M., Summo, C., & Caponio, F. (2021). Bioactive compounds from vine shoots, grape stalks, and wine lees: Their potential use in agro-food chains. Foods, 10(2), 1–16. https://doi.org/10.3390/foods10020342
Vernhet, A., & Moutounet, M. (2002). Fouling of organic microfiltration membranes by wine constituents: Importance, relative impact of wine polysccharides and polyphenols and incidence of membrane properties. Journal of Membrane Science, 201(1–2), 103–122. https://doi.org/10.1016/S0376-7388(01)00723-2
Vidal, S., Williams, P., Doco, T., Moutounet, M., & Pellerin, P. (2003). The polysaccharides of red wine: Total fractionation and characterization. Carbohydrate Polymers, 54(4), 439–447. https://doi.org/10.1016/S0144-8617(03)00152-8
Wang, Y., Singh, A. P., Hurst, W. J., Glinski, J. A., Koo, H., & Vorsa, N. (2016). Influence of degree-of-polymerization and linkage on the quantification of proanthocyanidins using 4-dimethylaminocinnamaldehyde (DMAC) assay. Journal of Agricultural and Food Chemistry, 64(11), 2190–2199. https://doi.org/10.1021/acs.jafc.5b05408
Zagklis, D. P., & Paraskeva, C. A. (2015). Purification of grape marc phenolic compounds through solvent extraction, membrane filtration and resin adsorption/desorption. Separation and Purification Technology, 156, 328–335. https://doi.org/10.1016/j.seppur.2015.10.019
Zhu, F., Du, B., Zheng, L., & Li, J. (2015). Advance on the bioactivity and potential applications of dietary fibre from grape pomace. Food Chemistry, 186, 207–212. https://doi.org/10.1016/j.foodchem.2014.07.057
Acknowledgements
Author J.A.A.M. gratefully acknowledges the CeFEMA / Instituto Superior Técnico /Universidade de Lisboa, and the Institute on Membrane Technology, ITM-CNR, University of Calabria, where he was guest scientists. J.A.A.M. is supported by a PhD fellowship from the University of Bologna (Italy).
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mejia, J.A.A., Ricci, A., Figueiredo, A.S. et al. Membrane-based Operations for the Fractionation of Polyphenols and Polysaccharides From Winery Sludges. Food Bioprocess Technol 15, 933–948 (2022). https://doi.org/10.1007/s11947-022-02795-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02795-3