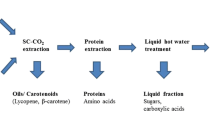
Abstract
Purpose
A large proportion of the European Union’s tomato crop is discarded during harvesting and there is a valorisation potential to recover proteins from this waste.
Methods
Cherry tomatoes were segregated into three separate components: juice, pomace (peels and skins), and seeds. The peels and skins, and seeds were separately hydrolyzed with carbohydrases to determine whether protein recovery could be increased. In addition, a strategy to fractionate the seeds was developed using sequential washing of milled tomato seeds, followed by low-speed centrifugation to remove the denser seed hulls and to collect the protein rich kernels remaining in suspension.
Results
The protein content of the seeds was highest with 27.4% while the peels and skins contained 7.6%. Carbohydrase mediated hydrolysis resulted in a minor increase in protein recovery of 10% from seeds using Filta 02L (cellulase, xylanase and β-glucanase), and the quantity of protein recovered from peels and skins increased by 210% using Tail 157 (pectinase, hemicellulase). The strategy to separate the seeds into two fractions, revealed that a higher proportion of the fibre (65%) was associated with the hull fraction compared with the original seeds (47%). A significant proportion of the fibre in this fraction was composed of lignin although the protein contents between both fractions was similar ranging from 27.4 to 29.9%.
Conclusions
These results reveal that carbohydrases were quite effective in protein extraction from peels and skins, but not from seeds. An alternative strategy was developed to remove the seed hulls from the milled seeds and to collect a fraction containing protein and dietary fibre where oil could be removed at this stage.
Graphical Abstract

Similar content being viewed by others
Statement of Novelty
Large quantities of waste tomatoes are produced yearly that have valorisation potential. These waste tomatoes were separated into three different components: juice, peels and skins, and seeds. Previous studies have focused on solvent extraction of oils from seeds prior to protein extraction, whereas this study focused on developing a process to extract a fraction enriched with fibre and protein without the initial de-oiling step thereby reducing solvent use.
Introduction
Tomatoes form a large proportion of the European Union’s fruit and vegetable crop harvest [1], with an annual production of 16.4 million tonnes. Approximately 0.5 million tonnes per annum is lost during the production stages [2], which is either used as animal feed or transferred to landfill sites. However, these losses may be based on indoor glasshouse production where pests, diseases and rates of growth can be controlled, whereas losses may be greater in some EU countries such as Spain that rely on outdoor growth [3]. There is an opportunity to valorize the unprocessed tomatoes or side residue streams generated from this crop, in order to isolate additional, value-added products. These include important compounds such as cutin found in the tomato skins [4] as a source of functional dietary fibre to reduce the risk of colorectal cancers [5] and carotenoids such as lycopene [6], with a range of medical related applications including in the prevention of certain cancers, cataracts and coronary diseases [7].
The current use for unsaleable or waste tomatoes is composting to form a fertilizer with a high protein content of up to 20% [8]. However, there is potential to valorize this biomass through fractionation to produce value added products that include functional proteins from this waste stream.
One of the challenges currently facing industry and ultimately society is the identification, production and utilisation of functionally useful plant proteins as alternatives to animal protein for applications in a range of sectors, especially as food ingredients. Central to this challenge is the development of cost-effective strategies for isolating plant proteins that retain structural conformations and consequently functional properties. Some of the emerging, alternative plant protein sources include pea, potato, canola and rice proteins but many more are required to fulfil the demand for the almost 50 different functional characteristics that are desired within the food market [9]. The wide range of application areas for functional plant proteins in the food industry is almost endless that includes ice creams, desserts, dressings, convenience produce, processed meat, bakery products and milk drinks.
Previous studies have focused on the potential source of functional seed protein containing 23–34% of material extracted from seeds that are separated from the tomato pulp by sedimentation [10,11,12]. The functional properties of these protein extracts showed higher oil absorption compared with soy proteins, while the foaming and emulsion characteristics were similar [13]. In laboratory feeding trials on rats, tomato seed protein resulted in acceptable weight gains but to a lesser extent compared with the use of casein [14]. One of the benefits of tomato seeds is the higher proportion of the essential amino acid, lysine, that could be used to fortify foods low in lysine, whereas other plant proteins such as soy and corn contain lower amounts of this amino acid [12]. While the potential functional properties of tomato seed protein have been reported, the development of an industrial process that does not rely on de-oiling of the tomato seeds prior to protein extraction has not been explored. Such a process would provide further progression towards developing a biorefinery process and associated value-chain approach to improve the economic viability of tomato waste valorisation, thereby diverting material away from animal feed applications or landfill.
The aim of this study was to develop a protocol to separate tomatoes into juice, pomace (pulp and peel), and seeds, with a view to ultimately incorporating these methods into a fruit and vegetable biorefinery, in order to create additional value to waste and/ or co-product streams. Compositional analysis was performed on each of these fractions to provide an insight into the valorisation potential and the presence of anti-nutritional compounds, the levels of which will impact on potential food applications areas because of reduced palatability. An assessment of the valorisation opportunities for these different fractions focused on proteins and involved determining the effectiveness of enzymatic hydrolysis to aid the separation process. Finally, a procedure was developed to separate the seed hulls and kernels into separate fractions.
Materials and Methods
Separation of Tomato Seeds
Cherry tomatoes (4.5 kg) that were deemed unsaleable were supplied frozen by Annecoop Ltd., Spain, which has one of the largest tomato farming operations in the EU aand supplies fresh produce to customers across Europe. These tomatoes were warmed at 50 °C for 5 min to loosen the skins and then passed through a juicing machine (Robot Coupe Automatic Sieve C200, Robot Coupe, France). Tomato juice was collected from one outlet and pomace was collected at the other. The pomace was placed into a 200 L pan, stirred and periodically passed through a sieve (5 mm diameter holes) and into a collection bucket. The pulp and skins collecting within the sieve were emptied whenever this became full and the seeds possessing a gelatinous outer layer passed through the holes of the sieve. Any tomato pulp or skins that had passed through the sieve that floated on the surface of the water in the collection bucket were skimmed off the surface. The collected seeds were then freeze dried to prevent degradation and ball milled.
Compositional Analysis of Tomato Components
The moisture content of each fraction was determined on a 5 g sample using a moisture analyser at 105 °C (Kern, Germany), until the evaporation rate was less than 2 mg of water/ 20 s. The total dry weights in the samples of the different tomato fruit fractions components were determined by multiplying the total wet weights by the percentage of dry weight that was determined using the moisture analyser. These dried samples were used to determine the fibre and phytic acid content. Fibre analysis was performed using a fibre analyser (Ankom Technology, USA) following the procedure described in the Ankom Technical Manual (http://www.ankom.com) for neutral detergent fibre (NDF) and acid detergent fibre (ADF), after extracting any residual oil in acetone by shaking ten times and incubating for 10 min. The acetone washed samples were air dried at room temperature and then oven dried at 103 °C for 30 min. NDF by treating fibre bags with 2 L neutral fibre detergent, 20 g sodium sulphite and 4 ml amylase (Ankom Technology) for 1 h at 100 °C and 10 psi and the fibre bags were washed three times with 2.67 ml amylase (Ankom Technology) in 116.67 ml deionized water for 5 min during each cycle. ADF was determined by treating the fibre bags with ADF solution for 1 h at 100 °C and 10 psi followed by 3 × 5 min washes with hot distilled water. Lignin analysis was performed in the fibre bags in the Daisy Incubator (Ankom Technology) at room temperature (3 h in 500 ml 72% sulphuric acid) and then continuously washed in tap water until the pH was neutral as determined using a pH meter. The Ankom bags were soaked in acetone for 5 min and dried at 103 °C for 3 h at the end of each treatment with NDF, ADF and lignin analysis. The ash contents were determined on 0.5 g of sample, not previously used for fibre content, at 600 °C for 4 h in a furnace oven. Hemicellulose content was determined by subtracting ADF from NDF values, cellulose content was determined by substracting lignin from ADF values, and lignin was determined by subtracting lignin from the weight of the empty bags. The ash contents in each of the respective fibre components were determined and subtracted from each component.
Protein determination was carried out by weighing wet material in duplicate to the equivalent of 1 g dry weight of material, as determined by mositure content. To the tubes containing pulp, 0.5 M NaOH (20 ml) was added, while 0.2 M NaOH (10 ml) was added to the seeds. The discrepency in concentration and volume used reflected the higher moisture content associated with the pulp. The tubes were incubated at 50 °C with end-over-end rotation (1 h) and then centrifuged at 1493 × g for 5 min. The supernatants were decanted into preweighed tubes and the weights of the supernatants were measured. The volumes were determined assuming that 1 g of supernatant was equivalent to 1 ml. The concentration of protein in the supernatants were determined using the Bradford assay and each sample was diluted until a reading of 0.4 was obtained, using the microplate reader at 595 nm.
The presence of anti-nutrients occurring as phytic acid was determined using the assay kit (Phytic Acid Kit, Megazyme, Ireland), which involved measuring free phosphates and then enzymatically released phosphates bound as phytic acids. The phytic acids were solublized from 1 g sample in 20 ml 0.66 M HCl. The assay contains phytase that degrades any phytic acids present into myo-inositol polyphosphates and another enzyme, alkaline phosphatase, into free phosphates. The phosphates are detected by reacting them with ammonium molybdate and acid to form a blue coloured compound that can be measured in the plate reader at 655 nm. The free phosphate concentrations were performed beforehand and these values were subtracted from the phosphates indicating phytic acids.
Enzyme Mediated Protein Extraction from Tomato Seeds
Freeze dried tomato seeds (20 g) were ball milled and samples of 100 mg samples were weighed into 2 ml Eppendorf tubes. To each tube, distilled water (1.65 ml) and carbohydrase (Table 1) diluted to one tenth (50 µl) was added and each treatment was performed in duplicate. The control consisted of distilled water (1.7 ml) without any enzyme. The milled seeds were resuspended in water and incubated at 50 °C with end-over-end rotation at 40 rpm (3 h). This temperature was chosen because it was within the optimum range for all of the carbohydrases as recommended specifications by the supplier (Tailorzymes) and enabled direct comparison of the carbohydrases without the influence of higher temperatures being a factor in higher activities. The tubes were centrifuged at 15,493×g (10 min) and the supernatants were collected noting the exact volume. The protein concentrations associated with each of the enzymes were determined and these values were subtracted from protein concentrations extracted with each of the enzymes. The protein concentrations were determined in the supernatants using the Bradford method and diluting the protein extracts until an absorbance at 595 nm reached an absorbance value of ~ 0.4.
Enzyme Mediated Extraction of Protein from Tomato Pulp
Wet tomato pulp (1 g equivalent dry weight) was centrifuged at 5575×g (10 min) and the supernatant was discarded. A minimal amount of protein was lost in the supernatant (0.057% of protein per g dry biomass). The pellets were resuspended in distilled water (20 ml) and the pH was adjusted from 4.2 to 4.7 ± 0.1 with 0.2 M NaOH. Each of the carbohydrases (Table 1) was separately added (50 µl) and the tubes were incubated with end-over-end rotation at 40 rpm for 3 h at 50 °C. The tubes were centrifuged at 5575×g for 5 min and the supernatant was decanted into another tube. The weights of the supernatants were measured, and protein concentrations were determined in water using the Bradford assay. Controls were also prepared using only carbohydrases at the same concentrations used to determine the effectiveness of carbohydrases in protein extraction and these values were subtracted from protein concentrations determined with each carbohydrase.
Measurement of Carbohydrase Activities
Cellulase activities were determined in duplicate for each carbohydrase by immersing 1 g Avicel in 10 ml deionized water (pH 6.1) and adding 50 µl carbohydrase. The tubes were incubated at 50 °C with end-over-end rotation at 40 rpm and then sugar concentrations were determined after 1 h incubation by centrifuging at 11337 xg (5 min). The quantity of sugars were measured after diluting a sample of the supernatant and then mixing at 1:3 with DNSA solution (1 g DNSA, 20 ml 2 M NaOH and 100 ml 20 g potassium sodium tartrate), heating to 100 °C for 5 min and then cooling on ice. The absorbance of these samples was measured at 540 nm against DNSA containing deionized water [15]. Xylanase activities were determined using 1 g oat xylan immersed in 10 ml deionized water (pH 5.7) using the same procedure described for measuring cellulase activity. A comparison was made between protein yields and enzyme activities because the enzymes were used as a liquid rather than as a solid. However, the protein contents of the carbohydrases were determined using the Bradford assay and specific enzyme activities were also calculated.
Laboratory Scale Protocol to Extract Proteins from Tomato Seeds
Duplicate sets of milled seeds (2.5 g) were transferred into 50 ml Falcon tubes and distilled water (40 ml) added to each. The tubes were shaken for 20 s and centrifuged for 3 min at either 40 × g or 1493 × g. The supernatants were decanted into fresh tubes and aliquots of 0.5 ml were taken to determine protein contents using the Bradford assay. Further extraction on the same samples involved adding distilled water (35 ml) to each pellet and the mixture was agitated (20 s), centrifuged (3 min at 40×g or 1493 × g and samples (0.5 ml) were collected from the supernatant for protein determination. The remainder of the supernatant was decanted and pooled with the previous supernatants. This whole step from resuspension of the pellet in 35 ml distilled water, to pooling of supernatants was repeated three more times. Ethanol {1:1 (v/v)} was added to the pooled supernatants, left overnight at 4 °C and the suspension was centrifuged at 5575×g for 5 min to collect the protein pellet. The remaining seed hulls and the extracted protein were freeze-dried and weighed. The non-fibrous portion of the seeds was calculated by subtracting the weights of the seed hulls and seed protein from the original weight of seeds at the start of the experiment.
Statistical Analysis
Levels of significant differences were calculated using Student’s t-Test on each processed sample compared with the control. Each enzyme treatment and the controls were performed in duplicate.
Gel Electrophoresis and Protein Identification
Protein samples were diluted (50 mg powder/ml) in 1 × lithium dodecyl sulfate sample buffer (Thermo Scientific) ± 10 mM reducing agent (dithiothreitol, DTT, Sigma-Aldrich). Samples were then heated to 70 °C for 30 min and centrifuged for 1 min at 10,000×g. Proteins were then separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 4–12% acrylamide gels (Genscript, Netherlands).
Protein bands of interest were excised with a scalpel and subjected to in-gel digestion using trypsin (Promega, USA). Extracted peptides were reduced and alkylated using DTT and iodoacetamide, respectively, and analyzed using matrix-assisted laser desorption ionization (MALDI) mass spectrometry (autoflex maX, Bruker Daltonics). The peptide mass list was searched against the NCBIProt database using Mascot search engine (Matrix Science, USA) with carbamidomethyl as a fixed modification of cysteine and oxidation of methionine as a variable modification.
Results and Discussion
Tomato Composition
The whole tomato fruits were fractionated into juice, pomace (pulp and peel), and seeds (Fig. 1). It was impossible to separate the peel and pulp because at this stage the peel appeared as fragmented pieces. The juice formed the largest proportion of material in tomatoes in terms of dry biomass (60%) and seeds (12%) were least. However, a large proportion of these tomatoes was comprised of water and only 49 g of tomato seeds were recovered from 4.5 kg fresh tomatoes. The processing of a larger quantity of round tomatoes (100 kg) resulted in a lower proportion of seeds being recovered (250 g) which would still be insufficient to perform pilot scale studies [Baker et al., unpublished]. Therefore, a different strategy using sedimentation of seeds was implemented to recover a larger quantity of material from a tomato juice processing plant [16]. It was impossible to separate the pulp and seeds from processed pomace using sieving because the gelatinous layer surrounding the seeds was removed during heat processing that would enable the seeds to pass through the sieve. The remaining pomace after processing resulted in the recovery of 44% of the dry biomass as seeds. However, the development of a strategy to recover crude protein from tomatoes that would be otherwise discarded is important process that should be independently investigated.
Compositional analysis of the pomace and seeds indicated that non-fibre was the major component in both fractions (Table 2), and a relatively high lignin content in the pomace (25.3 ± 1.5%). Whether the remaining material after fibre analysis was lignin is unclear and one possibility is that cutin, a macromolecule with properties similar to lignin, and that can form a structural component in mosses [17] may show acid resistant properties that are similar to lignin. In contrast, the lignin content in the seeds was considerably lower (9.51 ± 0.43%), with cellulose and lignin contents of the seeds having similar values to those reported previously [18]. There were significant quantities of cellulose in the pomace and of both hemicellulose and cellulose in the seeds, indicating that the application of an enzymatic processing step using carbohydrases could have an effect in releasing proteins from both fractions.
The protein content was determined for each of the tomato fractions, with the milled tomato seeds containing the highest amounts (Table 2). The protein content in un-milled seeds was 0.17 ± 0.02% and so it was necessary to mill the seeds to reduce particle size and facilitate optimised, downstream protein recovery [19]. Kjeldahl analysis revealed that the protein concentrations in milled seeds (Table 2) were similar to those reported previously [11, 12], but there are no reported values for the protein content of tomato pulp. It is possible that some of the protein remained inaccessible even under the alkaline treatment, resulting in lower values determined using the Bradford assay. Similar findings were revealed in another study, where protein determination of tomato seeds under weak alkaline conditions, using the Bradford assay, was lower than values determined using Kjeldahl analysis [20]. The Bradford assay measures soluble protein although the majority of soluble proteins will be determined at specific pH values [21]. In contrast, Kjeldahl analysis will also include insoluble proteins such as globular proteins although very low concentrations of other nitrogenous compounds besides amino acids may also be included.
Three quarters of phosphorus in plants is stored as phytates, although it cannot be absorbed by ruminants and the presence of phytates in food can lead to the deficiency in other minerals such as iron and zinc [22]. Our results showed that 76% of the total phosphorus was in the form of phytic acid and the amount was within the expected range for most cereal grains (Megazyme, Ireland). A previous study has shown that the carryover of phytates into the protein extract is much less compared with the quantity found in the original seeds [23].
Enzyme Mediated Protein Extraction from Seeds
A range of different enzyme mixtures were assessed in order to determine any improvements in protein extraction, each with minor differences in optimum temperature and pH conditions (Table 1). Enzyme mediated protein extraction from seeds indicated that the only carbohydrase which resulted in an increase in protein yield, following treatment, was Filta 02L. This was 10% higher and significantly different compared with the control (Fig. 2A) without enzyme (P = 0.008). Filta 02L possessed β-glucanase, xylanase and cellulase activities that would enable these carbohydrases to hydrolyse both the hemicellulose and cellulose present in the matrix. In contrast, Cellux 01L possessed β-glucanase and cellulase activities and did not show increased protein recovery. The other carbohydrases evaluated as part of this study, Tail 157 and Tail 01, did not possess cellulase activity and subsequently yielded lower quantities of protein compared with Filta 02L. Tail 113 which had similar carbohydrases to Filta 02L did not show increased levels compared with the control, perhaps due to lower levels of carbohydrase activity or possessed activity that targeted different carbohydrates. Therefore, carbohydrases that could completely degrade both hemicellulose and cellulose, such as xylanase, cellulase and β-glucanase appear to be necessary in order to increase the recovery of protein from tomato seeds. Some of the carbohydrases, Tail 157, Tail 113 and Cellux 01L, appeared to show a decrease in protein yield in comparison to the control without carbohydrase. However, only Tail 157 was significantly lower (P = 0.008) and it unclear why this resulted in a lower yield. It was apparent that this enzyme contained higher quantities of pectinases than the other enzymes and the release of galacturonate may form a gel-like substance in the presence of metallic ions [24], that may result in protein binding.
Protein yields based on total protein present as determined by Kjeldahl analysis of enzyme mediated extraction of A protein from tomato seeds and B protein from pulp, compared to control without enzymes (none). Maximum protein concentrations in mg per g biomass from wet seeds was 92.2 ± 2.7, from dry seeds was 97.8 ± 2.8, from wet pulp was 0.34 ± 0.3 and from dry pulp was 5.4 ± 0.5. Maximum protein yields compared with total alkaline extracted protein (which are lower than total protein based on Kjeldahl analysis) from tomato seeds was 123 ± 4% per g dry biomass and 38 ± 4% per g dry biommass
Enzyme Mediated Protein Extraction from Tomato Pulp
Protein extraction studies from the pulp indicated that Tail 157 increased the yield by 210%, which was significantly higher (P = 0.005) (Fig. 2B). The other enzymes also appeared to increase the protein yield between 30 and 40%, although only Tail 01 and Filta 02L were significantly higher (P < 0.05). Tail 157 appeared to act on the tomato peels causing them to completely disintegrate when whole tomatoes were incubated in the presence of this enzyme (unpublished). Considering the low levels of proteins that were extracted, it is important to include controls that account for the quantity of protein associated with each carbohydrase and these concentrations were subtracted from protein yields obtained with each carbohydrase. Tail 157 contains mostly pectinases, and the presence of a significant pectin content in tomatoes ranging from 5 to 10% of the total dry biomass [8] may be a factor in the effectiveness of this carbohydrase. Furthermore, the cellulase activity of Tail 157 at 94 ± 18 units per ml (Table 2) was considerably higher than the other carbohydrases where cellulose was a major fibre component (Table 3). Therefore, higher cellulase activity may contribute to higher protein recovery although further experiments would be required. There could be other factors such as physical treatments that could improve protein yields, including the use of microwave extraction from freeze dried tomato pulp to recover 7.8% of protein [25]. The remaining pulp could be valorised to recover carotenoids using solvent extraction [26].
Further Separation of the Milled Seeds
Another strategy was explored to separate the milled tomato seeds into two separate fractions, one containing predominately the seed hulls and the other fraction predominately containing the seed kernels. Tomato seeds are composed of ~ 50% non-fibrous material (Table 1), and it would appear, based on results from previous studies, that a significant amount of this soluble material could be tomato seed oil which has been found to comprise 31% of the total tomato seed weight [27]. Most studies have described the formation of tomato meal where the oils are removed through a defatting process by hexane extraction [11, 23, 28,29,30,31] or by using pressing [27]. However, the use of large quantities of organic solvent in pilot scale studies presents significant flammability issues, along with increasing CAPEX and OPEX and it is desirable to develop a green process that limits organic solvent use. Therefore, a process was investigated that would postpone the use of organic solvents until a later stage where lower volumes would be required due to the decrease in biomass.
The total protein yields after the first extraction and from the pooled sequential extraction mixtures was ~ 14% and 36% g based on total protein determined using Kjeldahl analysis (Fig. 3). Almost all of the alkali extractable protein was recovered as determined by the Bradford assay. This indicated that a significant amount of protein could be recovered from subsequent washes, although this does require the use of large volumes of water and ethanol. Rather than using large quantities of ethanol, protein could be precipitated using citric acid and up to 500 mg/l chitin as a coagulating agent [32] or different pH acidification ranges [33]. The volume of water to weight of biomass ratio at 18:1 during the initial wash was similar to previously reported values, conducted under dilute alkaline conditions [28]. However, a considerable amount of residual protein remained in the seed hull fraction following processing (Table 4). The quantity of protein in the crude protein extracts as determined by the different methods, Bradford and Kjeldahl analysis, were similar at ~ 30%, indicating that the majority of the protein was soluble and that a considerable proportion of other components were co-extracted with the protein. A hexane extraction revealed that 25% of the total crude protein was composed of oils and fats, indicating that the protein content would increase to ~ 40%. Further increases in protein yield could be obtained by ultrafiltration to collect proteins that remained in suspension even after ethanolic precipitation.
It was determined that the sequential aqueous extraction of proteins revealed that use of higher centrifugation speeds resulted in a lower proportion of material remaining suspended and more protein was associated with the centrifuged pellet which was assumed to contain seed hulls fragments based on appearance (Table 4). Higher levels of protein were recovered when lower speeds were used but the precipitates appeared slighter browner compared with precipitates obtained with higher centrifugation speeds due to the carryover of smaller seed hull fragments into this fraction. Nevertheless, there were minor differences between the crude fractions in terms of protein content determined using Kjeldahl analysis. The protein contents of the material left in the suspensions at different low speed centrifugation speeds were generally similar. Protein contents determined using Kjeldahl analysis were generally similar to those determined by the Bradford assay in protein extracts in contrast to differences between Kjeldahl and Bradford determined in the milled seeds. This might indicate that the proteins in this separated fraction were more accessible to the reagents in the Bradford assay whereas the majority of those in the seeds were inaccessible. Fibre analysis of the residual hulls indicated that the lignin content had increased more than three-fold compared with the original seeds, whereas the levels of hemicellulose and cellulose remained unchanged, and the non-fibre content decreased (Fig. 4). This clearly indicated a separation of the tomato seed components. The fraction containing the higher proportion of lignin contained a similar quantity of protein at 30% of the dry matter content to the protein extract, whereby this residual material could be evaluated as a potential ruminant by-product for animal or chicken feed (Fig. 5).
An investigation into alkali extracted tomato seed proteins indicated that 61% were salt soluble globulins and 37% were glutenin and gliadins that were soluble in acetic acid and ethanol, respectively [31]. Similarly, analysis of the protein composition of the seed fraction containing mostly seed kernels using SDS-PAGE (Fig. 6) showed that the major proteins were globulins based on comparison with previously published data [34]. Therefore, protein bands migrating in the reducing lane (+ DTT) at 49 kDa, 35 kDa and 20 kDa, represented globulins (Fig. 5). Another study showed that globulins precipitated at pH 3.8- 6.2, whereas those that were soluble proteins were precipitated at pH 3.5- 4.6 [35]. Therefore, it is possible that a higher proportion of soluble globulins were extracted under neutral pH conditions.
The fraction enriched with protein and fibre recovered in this study remained as a colloidal suspension but showed low solubility when precipitated and dried. However, the use of ultrafiltration has been shown to improve functional properties of proteins and this process could be combined with other physical pre-treatment processes such as microwaving for further enhancement [36]. One of the major challenges at this stage is upscaling from gram to kilogram scale quantities and understanding the caveats at larger scale to achieving the success determined at smaller scale.
Conclusions
In terms of enzyme efficiency, the use of carbohydrases showed a higher increase in protein yields from pulp and peel than from seeds compared to protein extraction without carbohydrases, despite the higher concentrations of hemicellulose in seeds. It would appear that under these conditions the use of carbohydrases might be ineffective from a technoeconomic perspective, due to the high production costs for these enzymes and when considering the minor increase in yield of protein from seeds and the lower quantity of protein associated with pulp and peel. However, a pre-process step was developed to remove a significant proportion of lignin from the milled seeds without the requirement for use of carbohydrases. This indicated that the other fraction contained a considerably lower lignin content and this milled seed fraction represented one third of the original weight of the seeds. Further processing to increase the protein content is required as part of the strategy for optimising and developing a commercial process to extract protein from seeds and to integrating this into a wider fruit and vegetable biorefinery approach.
Data Availability
Data is available on reasonable request.
References
Union Européenne. Commission européenne, & EUROSTAT: Agriculture, forestry and fishery statistics. Publications office of the European Union. https://doi.org/10.2785/143455 (2015)
Scherhaufer, S., Moates, G., Hartikainen, H., Waldron, K., Obersteiner, G.: Environmental impacts of food waste in Europe. Waste Manag. 77, 98–113 (2018). https://doi.org/10.1016/j.wasman.2018.04.038
Epp, M.: Tomato production varies in European countries. https://vegetablegrowersnews.com/article/tomato-production-varies-european-countries/https://doi.org/10.1021/jf00103a004 (2016)
Osman, S.F., Irwin, P., Fett, W.F., O’Connor, J.V., Parris, N.: Preparation, isolation, and characterization of cutin monomers and oligomers from tomato peels. J. Agric. Food Chem. 47(2), 799–802 (1999). https://doi.org/10.1021/jf980693r
Mudgil, D., Barak, S.: Classification, technological properties, and sustainable sources. In: Dietary Fiber: Properties, Recovery, and Applications, pp. 27–58. Academic Press. https://doi.org/10.1016/B978-0-12-816495-2.00002-2 (2019)
Choksi, P.M., Joshi, V.Y.: A review on lycopene—extraction, purification, stability and applications. Int. J. Food Prop. 10(2), 289–298 (2007). https://doi.org/10.1080/10942910601052699
Baysal, T., Ersus, S., Starmans, D.A.J.: Supercritical CO2 extraction of β-carotene and lycopene from tomato paste waste. J. Agric. Food Chem. 48(11), 5507–5511 (2000). https://doi.org/10.1021/jf000311t
Fritsch, C., Staebler, A., Happel, A., Cubero Márquez, M.A., Aguiló-Aguayo, I., Abadias, M., Gallur, M., Cigognini, I.M., Montanari, A., López, M.J., Suárez-Estrella, F.: Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: a review. Sustainability 9(8), 1492 (2017). https://doi.org/10.3390/su9081492
Akharume, F.U., Aluko, R.E., Adedeji, A.A.: Modification of plant proteins for improved functionality: a review. Compr. Rev. Food Sci. Food Saf. 20(1), 198–224 (2021). https://doi.org/10.1111/1541-4337.12688
Latlief, S.J., Knorr, D.: Tomato seed protein concentrates: effects of methods of recovery upon yield and compositional characteristics. J. Food Sci. 48(6), 1583–1586 (1983). https://doi.org/10.1111/j.1365-2621.1983.tb05036.x
Piyakina, G.A., Yunusov, T.S.: General characteristics of the proteins of tomato seed flour and tomato skin flour. Chem. Nat. Compd. 31(4), 495–499 (1995). https://doi.org/10.1007/BF01177421
Brodowski, D., Geisman, J.R.: Protein content and amino acid composition of protein of seeds from tomatoes at various stages of ripeness. J. Food Sci. 45(2), 228–229 (1980). https://doi.org/10.1111/j.1365-2621.1980.tb02582.x
Liadakis, G.N., Tzia, C., Oreopoulou, V., Thompoulos, C.D.: Isolation of tomato seed meal proteins with salt solutions. J. Food Sci. 63(3), 450–453 (1998). https://doi.org/10.1111/j.1365-2621.1998.tb15762.x
Sogi, D.S., Bhatia, R., Garg, S.K., Bawa, A.S.: Biological evaluation of tomato waste seed meals and protein concentrate. Food Chem. 89(1), 53–56 (2005). https://doi.org/10.1016/j.foodchem.2004.01.083
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959). https://doi.org/10.1021/ac60147a030
Miklavčič Višnjevec, A., Baker, P.W., Peeters, K., Schwarzkopf, M., Krienke, D., Charlton, A.: HPLC-DAD-qTOF compositional analysis of the phenolic compounds present in crude tomato protein extracts derived from food processing. Molecules (submitted)
Philippe, G., Sørensen, I., Jiao, C., Sun, X., Fei, Z., Domozych, D.S., Rose, J.K.: Cutin and suberin: assembly and origins of specialized lipidic cell wall scaffolds. Curr. Opin. Plant Biol. 55, 11–20 (2020). https://doi.org/10.1016/j.pbi.2020.01.008
Dalimov, D.N., Dalimova, G.N., Bhatt, M.: Chemical composition and lignins of tomato and pomegranate seeds. Chem. Nat. Compd. 39(1), 37–40 (2003). https://doi.org/10.1023/A:1024128512801
Seikova, I., Simeonov, E., Ivanova, E.: Protein leaching from tomato seed––experimental kinetics and prediction of effective diffusivity. J. Food Eng. 61(2), 165–171 (2004). https://doi.org/10.1016/S0260-8774(03)00083-9
Mechmeche, M., Kachouri, F., Ksontini, H., Hamdi, M.: Production of bioactive peptides from tomato seed isolate by Lactobacillus plantarum fermentation and enhancement of antioxidant activity. Food Biotechnol. 31(2), 94–113 (2017). https://doi.org/10.1080/08905436.2017.1302888
Douglas, F.W., Jr., Greenberg, R., Farrell, H.M., Jr., Edmondson, L.F.: Effects of ultra-high-temperature pasteurization on milk proteins. J. Agric. Food Chem. 29(1), 11–15 (1981)
Raboy, V.: Low phytic acid crops: observations based on four decades of research. Plants 9(2), 140 (2020). https://doi.org/10.3390/plants9020140
Sarkar, A., Kaul, P.: Evaluation of tomato processing by-products: a comparative study in a pilot scale setup. J. Food Process Eng. 37(3), 299–307 (2014). https://doi.org/10.1111/jfpe.12086
Maire du Poset, A., Lerbret, A., Boué, F., Zitolo, A., Assifaoui, A., Cousin, F.: Tuning the structure of galacturonate hydrogels: external gelation by Ca, Zn, or Fe cationic cross-linkers. Biomacromol 20(7), 2864–2872 (2019). https://doi.org/10.1021/acs.biomac.9b00726
Pinela, J., Prieto, M.A., Barreiro, M.F., Carvalho, A.M., Oliveira, M.B.P., Curran, T.P., Ferreira, I.C.: Valorisation of tomato wastes for development of nutrient-rich antioxidant ingredients: a sustainable approach towards the needs of the today’s society. Innov. Food Sci. Emerg. Technol. 41, 160–171 (2017). https://doi.org/10.1016/j.ifset.2017.02.004
Lu, Z., Wang, J., Gao, R., Ye, F., Zhao, G.: Sustainable valorisation of tomato pomace: a comprehensive review. Trends Food Sci. Technol. 86, 172–187 (2019). https://doi.org/10.1016/j.tifs.2019.02.020[27]
Zuorro, A., Lavecchia, R., Medici, F., Piga, L.: Enzyme-assisted production of tomato seed oil enriched with lycopene from tomato pomace. Food Bioprocess Technol. 6(12), 3499–3509 (2013). https://doi.org/10.1007/s11947-012-1003-6
Mechmeche, M., Kachouri, F., Chouabi, M., Ksontini, H., Setti, K., Hamdi, M.: Optimization of extraction parameters of protein isolate from tomato seed using response surface methodology. Food Anal. Methods 10(3), 809–819 (2017). https://doi.org/10.1007/s12161-016-0644-x
Meshginfar, N., Mahoonak, A.S., Hosseinian, F., Tsopmo, A.: Physicochemical, antioxidant, calcium binding, and angiotensin converting enzyme inhibitory properties of hydrolyzed tomato seed proteins. J. Food Biochem. 43(2), e12721 (2019). https://doi.org/10.1111/jfbc.12721
Sogi, D.S., Arora, M.S., Garg, S.K., Bawa, A.S.: Fractionation and electrophoresis of tomato waste seed proteins. Food Chem. 76(4), 449–454 (2002). https://doi.org/10.1016/S0308-8146(01)00304-1
Sogi, D.S., Garg, S.K., Bawa, A.S.: Functional properties of seed meals and protein concentrates from tomato-processing waste. J. Food Sci. 67(8), 2997–3001 (2002). https://doi.org/10.1111/j.1365-2621.2002.tb08850.x
Latlief, S.J., Knorr, D.: Effect of chitin as coagulating aid on protein yield, composition and functionality of tomato seed protein concentrates. J. Food Sci. 48(6), 1587–1590 (1983). https://doi.org/10.1111/j.1365-2621.1983.tb05037.x
Salikhova, S.R., Piyakina, G.A., Yunusov, T.S.: Proteins isolated from tomato seeds. Chem. Nat. Compd. 35(5), 572–574 (1999). https://doi.org/10.1016/j.foodhyd.2016.01.014
Sheoran, I.S., Olson, D.J.H., Ross, A.R.S., Sawhney, V.K.: Proteome analysis of embryo and endosperm from germinating tomato seeds. Proteomics 5(14), 3752–3764 (2005). https://doi.org/10.1002/pmic.200401209
Piyakina, G.A., Maksimov, V.V., Yunusov, T.S.: Some properties of the protein fractions of tomato seeds. Chem. Nat. Compd. 34(4), 492–495 (1998). https://doi.org/10.1007/BF02329603
Gençdağ, E., Görgüç, A., Yılmaz, F.M.: Recent advances in the recovery techniques of plant-based proteins from agro-industrial by-products. Food Rev. Intl. 37(4), 447–468 (2021). https://doi.org/10.1080/87559129.2019.1709203
Acknowledgements
The authors would like to thank the Bio Based Industries Joint Undertaking for providing funding for the Pro-enrich project (Grant Agreement No. 792050), under the European Union’s Horizon 2020 research and innovation programme. The authors are grateful to Nicolas Vidal (Anecoop Ltd, Spain) for co-ordinating the supply of whole cherry tomatoes, Simon Lausen (Tailorzymes Ltd. (Denmark) for provision of enzymes and sharing technical knowledge and the Food Technology Centre, Grŵp Llandrillo Menai, Gwynedd, Wales, for additional technical support.
Funding
Funding was provided by Horizon 2020 Framework Programme (Grant No. 792050).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baker, P.W., Preskett, D., Krienke, D. et al. Pre-processing Waste Tomatoes into Separated Streams with the Intention of Recovering Protein: Towards an Integrated Fruit and Vegetable Biorefinery Approach to Waste Minimization. Waste Biomass Valor 13, 3463–3473 (2022). https://doi.org/10.1007/s12649-022-01748-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01748-3