Abstract
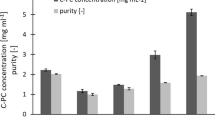
Spirulina platensis is a cyanobacterium with biological activities. This characteristic is mainly due to its blue pigment C-phycocyanin (C-PC), which is an important commercially available blue food colorant. To take maximum advantage of the benefits of C-PC, a comparative study of four different pretreatment techniques, namely, freeze–thaw (F/T), enzymatic (EE), ultrasound (US), and pulsed electric field (PEF) pretreatment, for C-PC extraction from S. platensis dry biomass was performed to select the method providing the highest yield and desired purity. The extraction of C-PC was conducted using 100 mM phosphate buffer, pH 6.8, which is a green solvent. The highest C-PC yield of 129.5 ± 7.78 mg/g was obtained with US pretreatment after 30 min of sonication at 40 kHz frequency. The next highest yield of 84.00 ± 2.13 mg/g was recorded after 240 μs of PEF pretreatment with a voltage of V = 24 kV/cm and 44 pulses (average pulse width of 5.36 μs). The highest purity of 2.47 ± 0.21 was obtained with F/T pretreatment, followed by US (2.15 ± 0.12) and PEF (2.13 ± 0.39).





Similar content being viewed by others
References
D.D. Hong, H.M. Hien, H.T. Anh, Studies on the analgesic and anti-inflammatory activities of Sargassum swartzii (Turner) C. Agardh (Phaeophyta) and Ulva reticulata Forsskal (Chlorophyta) in experiment animal models. Afr. J. Biotechnol. 10, 2308–2314 (2011)
S. Hu, X. Fana, P. Qib, X. Zhanga, Identification of anti-diabetes peptides from Spirulina platensis. J. Funct. Foods. 56, 333–341 (2019)
A. Silva, S.A. Silva, C. Lourenço-Lopes, C. Jimenez-Lopez, M. Carpena, P. Gullón, M. Fraga-Corral, V.F. Domingues, M. Fátima Barroso, J. Gandara, M. Prieto, Antibacterial use of macroalgae compounds against foodborne pathogens. J. Antibiot. Res. 9, 712 (2020)
R. Souza Cássio, M. Bezerra, P. Wallace, T. Souto Janeusa, Marine alkaloids with anti-inflammatory activity: current knowledge and future perspective. Mar. Drugs 18(3), 147 (2020)
M. Kuddus, P. Singh, G. Thomas, A. AlHazimi, Recent developments in production and biotechnological applications of C-phycocyanin. Biomed. Res. Int. 742–859 (2013)
R.F. Rizzo, B.N.C. Santos, G.F.P.S. Castro, T.S. Passo, M.A. Nascimento, H.D. Guerra, C.G. Silva, D.S. Dias, J.R. Domingues, K.G. Lima-Araújo, Production of phycobiliproteins by Arthrospira platensis under different light conditions for application in food products. J. Food Sci. Technol. 35(2), 247–252 (2015)
S.P. Cuellar-Bermudez, I. Aguilar-Hernandez, D.L. Cardenas-Chavez, N. Ornelas-Soto, M.A. Romero-Ogawa, R. Parra-Saldivar, Extraction and purification of high-value metabolites from microalgae: essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 8, 190–209 (2015)
I.N. Memije-Lazaroa, V. Blas-Valdiviaa, M. Franco-Colínb, Arthrospira maxima (Spirulina) and C-phycocyanin prevent the progression of chronic kidney disease and its cardiovascular complications. J. Funct. Foods 43, 37–43 (2018)
G. Martelli, C. Folli, L. Visai, M. Daglia, D. Ferrari, Thermal stability improvement of blue colorant C-phycocyanin from Spirulina platensis for food industry applications. Process Biochem. 49, 154–159 (2014)
S. Sekar, M. Chandramohan, Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J. Appl. Phycol. 20, 113–136 (2008)
A. Aouir, M. Amiali, T. Kirilova-Gachovska, A. Benchabane, A. Bitam, The effect of pulsed electric field (PEF) and ultrasoud (US) technologies on the extraction of phycopiliproteins from Arthrospira platensis, 2015 IEEE Canada International Humanitarian Technology Conference (IHTC2015), Ottawa (2015)
H.A. Tavanandi, R. Mittal, J. Chandrasekhar, K. Raghavarao, Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 31, 239–251 (2018)
H.A. Tavanandi, P. Vanjari, K. Raghavarao, Synergistic method for extraction of high purity Allophycocyanin from dry biomass of Arthrospira platensis and utilization of spent biomass for recovery of carotenoids. Sep. Purif. Technol. 225, 97–111 (2019)
F. Ruiz-Ruiz, E. López-Guajardo, P. Vázquez-Villegas, M.E. del Angel-Chong, K.D.P. Nigam, R.C. Willson, M. Rito-Palomares, Continuous aqueous two-phase extraction of microalgal C-phycocyanin using a coiled flow inverter. Chem. Eng. Process. 142, 107554 (2019)
S. Chittapun, V. Jonjaroen, K. Khumrangsee, T. Charoenrat, C-phycocyanin extraction from two freshwater cyanobacteria by freeze thaw and pulsed electric field techniques to improve extraction efficiency and purity. Algal Res. 46, 101789 (2020)
I. İlter, S. Akyıl, Z. Demirel, M. Koç, M. Conk-Dalay, F. Kaymak-Ertekin, Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J. Food Compos. Anal. 70, 78–88 (2018)
A.P.Q. Larrosa, A.S. Camara, J.M. Moura, L.A.A. Pinto, Spirulina sp. biomass dried/disrupted by different methods and their application in biofilms production. Food Sci. Biotechnol. 27, 1659–1665 (2018)
P. Ferreira-Santos, R. Nunes, F. De Biasio, G. Spigno, D. Gorgoglione, J.A. Teixeira, C.M.R. Rocha, Influence of thermal and electrical effects of ohmic heating on C-phycocyanin properties and biocomounds recovery from Spirulina platensis. LWT 128, 109491 (2020)
S. Akaberi, D. Krust, G. Muller, W. Frey, C. Gusbeth, Impact of incubation conditions on protein and C- phycocyanin recovery from Arthrospira platensis post- pulsed electric field treatment. Bioresour. Technol. 306, 123099 (2020)
S.P. Kamble, R.B. Gaikar, R.B. Padalia, K.D. Shinde, Extraction and purification of C-phycocyanin from dry Spirulina powder and evaluating its antioxidant, anticoagulation and prevention of DNA damage activity. J. Appl. Pharm. Sci. 3(08), 149–153 (2013)
S. Tavakoli, H. Hong, K. Wang, Q. Yang, H. Gahruie, H. Zhuang, Y. Luo, Ultrasonic-assisted food-grade solvent extraction of high-value added compounds from microalgae Spirulina platensis and evaluation of their antioxidant and antibacterial properties. Algal Res. 60, 102493 (2021)
R.W.M. Pott, The release of the blue biological pigment C-phycocyanin through calcium-aided cytolysis of live Spirulina sp. Color. Technol. 135(1), 17–21 (2019)
A. Kaferbock, S. Smetana, R. de Vos, C. Schwarz, S. Toepfl, O. Parniakov, Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Algal Res. 48, 101914 (2020)
J.M. Martínez, E. Luengo, G. Saldaña, I. Álvarez, J.C. Raso, phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 99, 1042–1047 (2017)
R. Sarada, M. Pillai, G. Ravishankar, Phycocyanin from Spirulina sp. influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 34(8), 795–801 (1999)
S. Saran, N. Puri, N. Jasuja, M. Kumar, G. Sharma, Optimization, purification and characterization of phycocyanin from Spirulina platensis. Int. J. Appl. Pure Sci. Agric. 2(3), 15–21 (2016)
M. Sobiechowska-Sasim, J. Stoń-Egiert, A. Kosakowska, Quantitative analysis of extracted phycobilin pigments in cyanobacteria—an assessment of spectrophotometric and spectrofluorometric methods. J. Appl. Phycol. 26(5), 2065–2074 (2014)
A.M. Goula, M. Ververi, A. Adamopoulou, K. Kaderides, Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 34, 821–830 (2017)
M. Amiali, O. Ngadi, Microbial decontamination of food by pulsed electric fields (PEFs). Microbial decontamination in the food industry. (Woodhead Publishing, 2012), pp. 407–449
O. Parniakov, O. Bals, V. Mykhailyk, N. Lebovka, E. Vorobiev, Unfreezable water in apple treated by pulsed electric fields: impact of osmotic impregnation in glycerol solutions. Food Bioprocess. Technol. 9, 243–251 (2016)
F. Bot, R. Verkerk, H. Mastwijk, M. Anese, V. Fogliano, E. Capuano, The effect of pulsed electric fields on carotenoids bioaccessibility: the role of tomato matrix. Food Chem. (2017)
F. Chemat, M.A. Vian, A.S. Fabiano-Tixier, M. Nutrizio, A.R. Jambrak, P.E. Munekata, G. Cravotto, A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 22(8), 2325–2353 (2020)
A.S. Peshkovsky, L. Peshkovsky, S. Bystryak, Scalable high-power ultrasonic technology for the production of translucent nanoemulsions. Chem. Eng. Process. 69, 77–82 (2013)
R.D.P. Rodrigues, F. de Castro, R. de Santiago-Aguiar, M. Valderez Ponte Rocha, Ultrasound-assisted extraction of phycobiliproteins from Spirulina (Arthrospira) platensis using protic ionic liquids as solvent. Algal Res. 31, 454–462 (2018)
L. Vernes, M. Abert-Vian, M. El Maâtaoui, Y. Tao, I. Bornard, F. Chemat, Application of ultrasound for green extraction of proteins from Spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultra Sonochem. 54, 48–60 (2019)
B.K. Tiwari, Ultrasound: a clean, green extraction technology. Trends. Anal. Chem. 71, 100–109 (2015)
M. Goettel, C. Eing, C. Gusbeth, R. Straessner, W. Frey, Pulsed electric field assisted extraction of intracellular valuables from microalgae. Algal Res. 2(4), 401–408 (2013)
O. Martın-Belloso, R. Soliva-Fortuny, Pulsed electric fields processing basics. Nonthermal processing technologies for food. 155–175 (2011)
N. Lebovka, E. Vorobiev, F. Chemat, Enhancing Extraction Processes in the Food Industry (CRC Press, Boca Raton, 2016)
E. Luengo, S. Condón-Abanto, I. Álvarez, J. Raso, Effect of pulsed electric field treatments on permeabilization and extraction of pigments from Chlorella vulgaris. J. Membr. Biol. 247(12), 1269–1277 (2014)
R. Zhang, O. Parniakov, N. Grimi, N. Lebovka, L. Marchal, E. Vorobiev, Emerging techniques for cell disruption and extraction of valuable bio-molecules of microalgae Nannochloropsis sp. Biopro. and Biosyst. Eng. (2018)
A.A. Jerley, D.M. Prabu, Purification, characterization and antioxidant properties of C-Phycocyanin from Spirulina platensis. SIRJ-APBBP. 1, 7–15 (2015)
J. Vocadlo, G.J. Davies, R. Laine, S.G. Withers, Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature 412(6849), 835–838 (2001)
T. K Gachovska, S. Kumar, H. Thippareddi, F. Williams, in Ultraviolet and Pulsed Electric Field Treatments Have Additive Effect on Inactivation of E. coli in Apple Juice, ed. by P. F. (Paul Frazer) (Williams Publicationsm, 2008), p. 47
M. Rito-Palomares, L. Nunez, D. Amador, Practical application of aqueous two-phase systems for the development of a prototype process for c-phycocyanin recovery from Spirulina maxima. J. Chem. Technol. Biotechnol. 76(12), 1273–1280 (2001)
S.T. Silveira, J.D.M. Burkert, J.A.V. Costa, C.A.V. Burkert, S.J. Kalil, Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 98(8), 1629–1634 (2007)
S. Boussiba, A. Richmond, Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch. Microbiol. 120(2), 155–159 (1979)
G. Prabakaran, P. Sampathkumar, M. Kavisri, M. Moovendhan, Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and anti inflammatory effect. Int. J. Biol. Macromol. 153, 256–263 (2020)
I. Chentir, M. Hamdi, S. Li, A. Doumandji, G. Markou, M. Nasri, Stability, bio- functionality and bio- activity of crude phycocyanin from a two-phase cultured Saharian Arthrospira sp. strain. Algal. Res. 35, 395–406 (2018)
J. Da Costa Ores, M.C.A. de Amarante, S.J. Kalil, Co-production of carbonic anhydrase and phycobiliproteins by Spirulina sp. and Synechococcus nidulans. Bioresour. Technol. 219, 219–227 (2016)
K. Nakagawa, W. Ritcharoen, P. Sri-Uam, P. Pavasant, S. Adachi, Antioxidant properties of convective-air-dried Spirulina maxima: Evaluation of phycocyanin retention by a simple mathematical model of air-drying. Food Bioprod. Process. 100, 292–302 (2016)
C.C. Moraes, L. Sala, G.P. Cerveira, S.J. Kalil, C-phycocyanin extraction from Spirulina platensis wet biomass. Braz. J. Chem. Eng. 28, 45–49 (2011)
C. Fratelli, M. Burck, M.C.A. de Amarante, A.R.C. Braga, Antioxidant potential of nature's “something blue”: something new in the marriage of biological activity and extraction methods applied to C-phycocyanin. Trends Food Sci. Technol. (2020)
D.J. Yu, J.Y. Hwang, S.W. Chung, H.D. Oh, S.K. Yun, H.J. Lee, Changes in cold hardiness and carbohydrate content in peach (Prunus persica) trunk bark and wood tissues during cold acclimation and deacclimation. Sci. Hortic. 219, 45–52 (2017)
C. Ibáñez, A. Valdés, V. García-Cañas, C. Simó, M. Celebier, L. Rocamora-Reverte et al., Global Foodomics strategy to investigate the health benefits of dietary constituents. J. Chromatogr. A 1248, 139–153 (2012)
M. Kissoudi, I. Sarakatsianos, V. Samanidou. P47: extraction, purification and evaluation of food-grade phycocyanin from Spirulina platensis. (2017)
A.C. Devi, H.A. Tavanandi, K. Govindaraju, K. Raghavarao, An effective method for extraction of high purity phycocyanins (C-PC and A-PC) from dry biomass of Arthrospira maxima. J. Appl. Phycol. 32, 1141–1151 (2020)
L. Vernès, P. Granvillain, F. Chemat, M. Vian, Phycocyanin from Arthrospira platensis. Production, extraction and analysis. Curr. Biotechnol. 4, 481–491 (2015)
M. Marić, A.N. Grassino, Z. Zhu, F.J. Barba, M. Brnčić, S.R. Brnčić, An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 76, 28–37 (2018)
Y. Zhang, X. Kong, Z. Wang, Y. Sun, S. Zhu, L. Li, P. Lv, Optimization of enzymatic hydrolysis for effective lipid extraction from microalgae Scenedesmus sp. Renew. Energy 125, 1049–1057 (2018)
W. Pan-utai, S. Iamtham, Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem. 82, 189–198 (2019)
M.B. Bachchhav, M.V. Kulkarni, A.G. Ingale, Process-intensified extraction of phycocyanin followed by β-carotene from Spirulina platensis using ultrasound-assisted extraction. Sep. Sci. Technol. 55, 932–944 (2020)
H. Hadiyanto, S. Suttrisnorhadi, Response surface optimization of ultrasound assisted extraction (UAE) of phycocyanin from microalgae Spirulina platensis. Emir. J. Food Agric. 227–234 (2016)
M. Gorgich, M.L. Passos, T.M. Mata, A.A. Martins, M.L.M. Saraiva, N.S. Caetano, Enhancing extraction and purification of phycocyanin from Arthrospira sp. with lower energy consumption. Energy Rep. 6, 312–318 (2020)
R. Vali Aftari, K. Rezaei, A. Mortazavi, A.R. Bandani, The optimized concentration and purity of Spirulina platensis C-phycocyanin: a comparative study on microwave-assisted and ultrasound-assisted extraction methods. J. Food Process. Preserv. 39(6), 3080–3091 (2015)
H.A. Tavanandi, K. Raghavarao, Ultrasound-assisted enzymatic extraction of natural food colorant C-Phycocyanin from dry biomass of Arthrospira platensis. Lebensm Wiss Technol. 118, 108802 (2020)
J.M. Martínez, Z. Gojkovic, L. Ferro, M. Maza, I. Álvarez, J. Raso, C. Funk, Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour. Technol. 289, 121694 (2019)
D.P. Jaeschke, G.D. Mercali, L.D.F. Marczak, G. Müller, W. Frey, C. Gusbeth, Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 283, 207–212 (2019)
S. Asavasanti, W. Ristenpart, P. Stroeve, D.M. Barrett, Permeabilization of plant tissues by monopolar pulsed electric fields: effect of frequency. J. Food Sci. 76, 98–111 (2011)
N. Grimi, A. Dubois, L. Marchal, S. Jubeau, N. Lebovka, E. Vorobiev, Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresour. Technol. 153, 254–259 (2014)
E. Günerken, E. D’Hondt, M.H.M. Eppink, L. Garcia-Gonzalez, K. Elst, R.H. Wijffels, Cell disruption for microalgae biorefineries. Biotechnol. Adv. 33, 243–260 (2015)
D. Carullo, F. Donsì, G. Ferrari, G. Pataro, Extraction improvement of water-soluble compounds from Arthrospira platensis through the combination of high-shear homogenization and pulsed electric fields. Algal. Res. 57, 102341 (2021)
Acknowledgements
The authors thank the Scientific Research and Technological Development (DGRSDT) of Algeria for financial support and the staff of the Food Technology and Human Nutrition Laboratory (LRTANH-ENSA) for technical assistance.
Author information
Authors and Affiliations
Contributions
NEHB: Investigation, Formal analysis, Validation, Writing—original draft. FSA: Methodology, Investigation. AB: Doctoral supervision committee., Project administration. IS: Methodology, Investigation. AB: Writing- review, Doctoral supervision committee. TG: PEF conception, Methodology, Writing- review. MA: Conceptualization, Supervision, Review & editing, Funding acquisition, Doctoral supervision committee, Director of Research Laboratory of Food Technology and Human Nutrition (LRTANH).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berrouane, N.E.H., Attal, FS., Benchabane, A. et al. Freeze–thaw-, enzyme-, ultrasound- and pulsed electric field-assisted extractions of C-phycocyanin from Spirulina platensis dry biomass. Food Measure 16, 1625–1635 (2022). https://doi.org/10.1007/s11694-021-01264-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01264-3