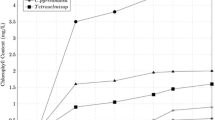
Abstract
Unearthing new sustainable and economically viable sources for biofuel production which do not affect the environment is a dire need of the hour. Microalgae is one such promising source due to its high lipid content, productivity, and carbon neutrality. Identification of appropriate strain and process optimization decides the biomass productivity, nutrient value, and oil content which are the major factors for commercialization. In the present work, mass cultivation of halophilic Aphanothece halophytica in raceway ponds was optimized by using organic and inorganic nutrients by using design of experiments. Organic flocculant, neem plus was successfully adapted for harvesting the biomass and oil extraction was done with solvent methodology. A maximum lipid yield of 29.3% was obtained on wet basis, when the reaction temperature, reaction time, biomass-to-solvent ratio and mixing intensity were kept at 68 ºC, 190 min, 9:1, and 300 rpm respectively. Similarly, on dry basis, a lipid yield of 27.5% was reported when the reaction temperature, reaction time, biomass-to-solvent ratio and mixing intensity were maintained at 68 ºC, 190 min, 12:1, and 300 rpm respectively. GC–MS analysis of the lipid was done to appropriate the combination of fatty acid for enhancing the biofuel production.





Similar content being viewed by others
References
Akubude VC, Nwaigwe KN, Dintwa E (2019) Production of biodiesel from microalgae via nanocatalyzed transesterification process: a review. Mater Sci Technol 2:216–225. https://doi.org/10.1016/j.mset.2018.12.006
Capson-Tojo G, Torres A, Munoz R, Bartacek J, Jeison D (2017) Mesophilic and thermophilic anaerobic digestion of lipid-extracted microalgae N-gaditana for methane production Renew. Energ 105:539–546. https://doi.org/10.1016/j.renene.2016.12.052
Kings AJ, Raj RE, Miriam LRM, Visvanathan MA (2017) Growth studies on microalgae Euglena sanguinea in various natural eco-friendly composite media to optimize the lipid productivity. Bioresour Technol 244:1349–1357. https://doi.org/10.1016/j.biortech.2017.06.136
Zhang Y, Kang X, Wang Z, Kong X, Li L, Sun Y, Zhu S, Feng S, Luo X, Lv P (2018) Enhancement of the energy yield from microalgae via enzymatic pretreatment and anaerobic codigestion. Energy 164:400–407. https://doi.org/10.1016/j.energy.2018.08.124
Mubarak M, Shaija A, Suchithra TV (2015) A review on the extraction of lipid from microalgae for biodiesel production. Algal Res 7:117–123. https://doi.org/10.1016/j.algal.2014.10.008
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30:709–732. https://doi.org/10.1016/j.biotechadv.2012.01.001
Surkatti R, Al-Zuhair S (2018) Effect of cresols treatment by microalgae on the cells composition. J of Water Process Eng 26:250–256. https://doi.org/10.1016/j.jwpe.2018.10.022
Medina AR, Grima EM, Gimenez AG, Gonzalez MJ (1998) Downstream processing of algal polyunsaturated fatty acids. Biotechnol Adv 16:517–580. https://doi.org/10.1016/S0734-9750(97)00083-9
Pragya N, Pandey KK, Sahoo PK (2013) A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew Sust Energ Rev 24:159–171. https://doi.org/10.1016/j.rser.2013.03.034
Iverson SJ, Lang SL, Cooper MH (2011) Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 36(11):1283–1287. https://doi.org/10.1007/s11745-001-0843-0
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/y59-099
Selvakumar P, Sivashanmugam P (2019) Ultrasound assisted oleaginous yeast lipid extraction and garbage lipasecatalyzed transesterification for enhanced biodiesel production. Energy Convers Manag 179:141–151. https://doi.org/10.1016/j.enconman.2018.10.051
Selvakumar P, Sivashanmugam P (2018) Study on lipid accumulation in novel oleaginous yeast Naganishialiquefaciens NITTS2 utilizing pre-digested municipal waste activated sludge: a low-cost feedstock for biodiesel production. Appl Biochem Biotechnol 186(3):731–749. https://doi.org/10.1007/s12010-018-2777-4
Dejoye C, Vian MA, Lumia G, Bouscarle C, Charton F, Chemat F (2011) Combined extraction processes of lipid from Chlorella vulgaris microalgae: microwave prior to supercritical carbon dioxide extraction. Int J Mol Sci 12:9332–9341. https://doi.org/10.3390/ijms12129332
Kumar SJ, Kumar GV, Dash A, Scholz P, Banerjee R (2017) Sustainable green solvents and techniques for lipid extraction from microalgae: a review. Algal Res 1:138–147. https://doi.org/10.1016/j.algal.2016.11.014
Al-Ameri M, Al-Zuhair S (2018) Using switchable solvents for enhanced, simultaneous microalgae oil extraction-reaction for biodiesel production. Biochem Eng J 141:217–224. https://doi.org/10.1016/j.bej.2018.10.017
Dhandayuthapani K, SenthilKumar P, ChiaWY CKW, Karthik V, Selvarangaraj H, Selvakumar P, Sivashanmugam P, LokeShow P (2021) Bioethanol from hydrolysate of ultrasonic processed robust microalgal biomass cultivated in dairy wastewater under optimal strategy. Energy 244:122604. https://doi.org/10.1016/j.energy.2021.122604
Kavitha S, Gajendran T, Saranya K, Selvakumar P, Manivasagan V (2021) Study on consolidated bioprocessing of pre-treated Nannochloropsis gaditana biomass into ethanol under optimal strategy. Renew Energy 172:440–452. https://doi.org/10.1016/j.renene.2021.03.015
Dhandayuthapani K, Sarumathi V, Selvakumar P, Temesgen T, Asaithambi P, Sivashanmugam P (2021) Study on the ethanol production from hydrolysate derived by ultrasonic pretreated defatted biomass of chlorella sorokiniana NITTS3. Chem Data Coll 31:100641. https://doi.org/10.1016/j.cdc.2020.100641
Shyam KP, Rajkumar P, Ramya V, Miriam LM (2021) Biorefining polyvinyl alcohol (PVA) by Enterobacter cloacae and its polyhydroxy butyrate (PHB) production ability. Ind Biotechnol 17(2):92–99. https://doi.org/10.1089/ind.2020.0039
Srinidhi C, Madhusudhan A, Channapattana SV, Gawali SV, Aithal K (2021) RSM based parameter optimization of CI engine fuelled with nickel oxide dosed Azadirachta indica methyl ester. Energy 234:121282. https://doi.org/10.1016/j.energy.2021.121282
Shyam KP, Rajkumar P, Ramya V, Sivabalan S, Kings AJ, Miriam LM (2021) Exopolysaccharide production by optimized medium using novel marine Enterobacter cloacae MBB8 isolate and its antioxidant potential. Carbohydr Polym Technol Appl 2:100070. https://doi.org/10.1016/j.carpta.2021.100070
Miriam LRM, Raj RE, Kings AJ, Visvanathan MA (2017) Identification and characterization of a novel biodiesel producing halophilic Aphanothece halophytica and its growth and lipid optimization in various media. Energy Convers Manag 141:93–100. https://doi.org/10.1016/j.enconman.2016.05.041
Venkatesan R, Karuppiah PS, Arumugam G, Balamuthu K (2019) β-Asarone exhibits antifungal activity by inhibiting ergosterol biosynthesis in Aspergillus niger ATCC 16888. Proc Natl Acad Sci India Sect B Biol Sci 89(1):173–184. https://doi.org/10.1007/s40011-017-0930-4
Siham D, Djamal Z, Luveshan R, Ismail R, Faizal B (2016) Cultivation of Chlorella pyrenoidosa in outdoor open raceway pond using domestic wastewater as medium in arid desert region. Bioresour Technol 219:749–752. https://doi.org/10.1016/j.biortech.2016.08.019
Suparmaniam U, Lam MK, Uemura Y, Lim JW, Lee KT, Shuit SH (2019) Insights into the microalgae cultivation technology and harvesting process for biofuel production: a review. Renew Sust Energy Rev 115:109361. https://doi.org/10.1016/j.rser.2019.109361
Chokshi K, Pancha I, Ghosh A, Mishra S (2017) Salinity induced oxidative stress alters the physiological responses and improves the biofuel potential of green microalgae Acutodesmus dimorphus. Bioresour Technol 244:1376–1383. https://doi.org/10.1016/j.biortech.2017.05.003
Chen B, Wan C, Mehmood MA, Chang JS, Bai F, Zhao X (2017) Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products: a review. Bioresour Technol 244:1198–1206. https://doi.org/10.1016/j.biortech.2017.05.170
Yin Z, Zhu L, Li S, Hu T, Chu R, Mo F, Hu D, Liu C, Li B (2020) A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: environmental pollution control and future directions. Bioresour Technol 301:122804. https://doi.org/10.1016/j.biortech.2020.122804
Kumar AV, Agila E, Sivakumar P, Salam Z, Rengasmy R, Ani FN (2014) Optimization and characterization of biodiesel production from microalgae Botryococcus grown at semi-continuous system. Energ Convers Manag 88:936–946. https://doi.org/10.1016/j.enconman.2014.09.019
Rashid N, Rehman MSW, Sadiq M, Mahmood T, Han JI (2014) Current status, issues and developments in microalgae derived biodiesel production. Renew Sustain Ener Rev 40:760–778. https://doi.org/10.1016/j.rser.2014.07.104
Letelier-Gordo CO, Holdt SL, De-Francisci D, Karakashev DB, Angelidaki I (2014) Effective harvesting of the microalgae Chlorella protothecoides via bioflocculation with cationic starch. Bioresour Technol 167:214–218. https://doi.org/10.1016/j.biortech.2014.06.014
Dharma S, Masjuki HH, Ong HC, Sebayang AH, Silitonga AS, Kusumo F, Mahlia TMI (2016) Optimization of biodiesel production process for mixed Jatropha curcas – Ceiba pentandra biodiesel using response surface methodology. Energ Convers Manag 115:178–190. https://doi.org/10.1016/j.enconman.2016.02.034
Lakshmikandan M, Murugesan AG, Wang S, Abomohra AEF, Jovita PA, Kiruthiga S (2019) Sustainable biomass production under CO2 conditions and effective wet microalgae lipid extraction for biodiesel production. J Clean Prod 247:119398. https://doi.org/10.1016/j.jclepro.2019.119398
Chen J, Li J, Dong W, Zhang X, Tyagi RD, Drogui P, Surampalli RY (2018) The potential of microalgae in biodiesel production. Renew Sustain Energy Rev 90:336–346. https://doi.org/10.1016/j.rser.2017.07.044
Hosseini NS, Shang H, Scott JA (2018) Optimization of microalgae sourced lipids production for biodiesel in a top-lit gas-lift bioreactor using response surface methodology. Energy 146:47–56. https://doi.org/10.1016/j.energy.2017.08.085
Kadi H, Fellag H (2001) Modelling of oil extraction from olive foot cake using hexane. Grasas Aceites 52:369–372. https://doi.org/10.3989/gya.2001.v52.i6.346
Pokoo-Aikins G, Heath A, Mentzer RA, Mannan SM, Rogers WJ, El-Halwagi MM (2010) A multi-criteria approach to screening alternatives for converting sewage sludge to biodiesel. J of Loss Prev Process Ind 23:412–420. https://doi.org/10.1016/j.jlp.2010.01.005
Zhang L, Cheng J, Pei H, Pan JL, Hou Q, Han F (2018) Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renew Ener 115:276–287. https://doi.org/10.1016/j.renene.2017.08.034
Jesus SSD, Ferreira GF, Moreira LS, Maciel MRW, Filho RM (2019) Comparison of several methods for effective lipid extraction from wet microalgae using green solvents. Renew Ener 43:130–141. https://doi.org/10.1016/j.renene.2019.04.168
Patil PD, Gude VG, Mannarswamy A, Deng S, Cooke P, Munson-McGee S, Rhodes I, Lammers P, Nirmalakhandan N (2011) Optimization of direct conversion of wet algae to biodiesel under supercritical methanol conditions. Bioresour Technol 102:118–122. https://doi.org/10.1016/j.biortech.2010.06.031
Zhou X, Jin W, Wang Q, Guo S, Tu R, Han S, Chen C, Xie G, Qu F, Wang Q (2019) Enhancement of productivity of Chlorella pyrenoidosa lipids for biodiesel using co-culture with ammonia-oxidizing bacteria in municipal wastewater. Renew Ener 151:598–603. https://doi.org/10.1016/j.renene.2019.11.063
Lee SJ, Yoon BD, Oh HM (1998) Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol Tech 12:553–556. https://doi.org/10.1023/A:1008811716448
Rajak U, Nashine P, Verma TN (2018) Assessment of diesel engine performance using spirulina microalgae biodiesel. Energy 166:1025–1036. https://doi.org/10.1016/j.energy.2018.10.098
Channapattana SV, Pawar AA, Gawali SV, Hole J (2020) The effect of nickel oxide nano-additives in Azadirachta indica biodiesel diesel blend on engine performance and emission characteristics by varying compression ratio. Environ Prog Sustain Energy 40(2):13514. https://doi.org/10.1016/10.1002/ep.13514
Miriam LRM, Raj RE, Kings AJ, Visvanathan MA (2017) Enhanced FAME production using green catalyst with superior profile from the isolated halophilic Aphanothece halophytica grown in raceway ponds. Energy Convers Manag 151:63–72. https://doi.org/10.1016/j.enconman.2017.08.071
Srinidhi C, Madhusudhan A, Channapattana SV, Gawali SV (2020) Comparitive investigation of performance and emission features of methanol, ethanol, DEE, and nanopartilces as fuel additives in diesel-biodiesel blends. Heat Transfer 50(29):1–19. https://doi.org/10.1002/htj.21997
Kings AJ, Raj RE, Miriam LRM, Visvanathan MA (2017) Cultivation, extraction and optimization of biodiesel production from potential microalgae Euglena sanguinea using eco-friendly natural catalyst. Energy Convers Manag 141:224–235. https://doi.org/10.1016/j.enconman.2016.08.018
Lani NS, Ngadi N, Yahya NNY, Rahman RA (2017) Synthesis, characterization and performance of silica impregnated calcium oxide as heterogeneous catalyst in biodiesel production. J Clean Prod 146:116–124. https://doi.org/10.1016/j.jclepro.2016.06.058
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monisha Miriam, L.R., Kings, A.J., Raj, R.E. et al. Process Optimization of Lipid Extraction from Microalgae Aphanothece halophytica in Wet and Dry Conditions. Bioenerg. Res. 16, 1051–1064 (2023). https://doi.org/10.1007/s12155-022-10464-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10464-8