Abstract
This work evaluates the purification of melon peel extracts obtained by two eco-friendly methods: autohydrolysis and sodium acetate/urea/water extraction (1:3:1.6), an alkaline deep eutectic solvent (DES). For that, sequential ethanol precipitation and resin adsorption/desorption stages were proposed for the separate recovery of the pectic and phenolic fractions. In order to screen the optimal purification conditions, in a first step, the effect of ethanol concentrations (from 70 to 85%) on the precipitation of pectic oligosaccharides was assayed. Subsequently, the influence of the selected resin (Amberlite XAD4, XAD16HP and XAD7HP), liquid/resin ratios, and desorption sequences (varying ethanol concentrations and pH) on the phenolic compounds was also studied. The highest pectin yields were achieved with 85% ethanol: 16.11 and 18.05 g pectin/100 g water-insoluble solids (WIS) for autohydrolysis and DES extracts, respectively. All pectins presented a galacturonic acid content of about 45%, while autohydrolysis pectin presented a higher amount of neutral sugar side chains. The presence of low methoxyl GalA and both linear and branched OGalA with DP from 2 to 20 was also confirmed by FTIR and HPAEC-PAD analysis, respectively. Concerning the phenolic fraction, the resin adsorption and desorption steps at the selected conditions (XAD4 resin, liquid/resin ratio of 2 mL/g, eluted with 50% ethanol thrice) resulted in 79.55 and 4.08 mg GAE/g non-volatile content (NVC) for autohydrolysis and DES extracts, respectively, with improved antioxidant capacity. Moreover, some phenolic acids (protocatechuic and ferulic acids) and flavonoids (orientin, vitexin and naringenin) were quantified in the extracts by HPLC–PDA-MS/MS.
Similar content being viewed by others
Introduction
Melon (Cucumis melo L.) is a crop extensively cultivated worldwide due to its capacity to adapt to different climates. This fruit, in addition to presenting a high nutritional value, is considered a source of bioactive compounds that provide health benefits and reduce the risks of illnesses such as cancer or cardiovascular diseases (FAO, 2021; Miller et al., 2020; Rolim et al., 2019; Silva et al., 2020). The industrial processing of melon generates high amounts of peels and seeds as by-products, representing about one third of the weight of the fruit (Rico et al., 2020a). These by-products could be valorized since they are considered an abundant and low-cost source of bioactive natural molecules such as polyphenols, pectin polysaccharides, fatty acids or proteins (Gómez-García et al., 2021b; Rico et al., 2020a; Silva et al., 2020).
Pectin is a complex polysaccharide that presents homogalacturonan (HG), rhamnogalacturonan I (RG-I) and rhamnogalacturonan II (RG-II) in its structure (Kumar et al., 2020; Mao et al., 2019a). HG accounts for about 65% of pectin in plants and is made of a linear backbone of galacturonic acid (GalA) that is characterized with partial methyl esterification at the carboxyl groups at C-6 (determining the degree of methyl esterification, DM) and acetyl-esterification at O-2 or O-3 (degree of acetylation, DA). RG-I represents 20–35% of pectin and is composed of GalA and rhamnose (Rha) disaccharides with side chains made of galactose (Gal) and arabinose (Ara) units. RG-II accounts for less than 10% of pectin, and it is heavily branched with 12 types of glycosyl residues linked by at least 22 types of glycosidic bonds (Li et al., 2021; Mao et al., 2019a; Thibault & Ralet, 2003). The structure and composition of the pectin and its content in HG, RG-I, and RG-II determine its properties and applications (Huang et al., 2021), and these parameters can be modulated by the selection of the extraction technology. For instance, pectins with a high content in neutral sugar side chains have shown higher prebiotic activity (Kumar et al., 2020; Mao et al., 2019a), whereas more linear pectins are believed to possess higher viscosity, which improves film formation (Huang et al., 2021).
Phenolic compounds are a wide group of secondary plant metabolites with important health benefits due to their antioxidant, antimicrobial, anti-inflammatory, and antidiabetic activities (Ding et al., 2020; Rolim et al., 2019; Silva et al., 2020). Countless works have been published exploring the extraction of free phenolic compounds from plant matrixes, with less focus on non-extractable polyphenols, which are bound to macromolecules such as polysaccharides or proteins and therefore they cannot be recovered by traditional aqueous-organic extractions (Ding et al., 2020; Dzah et al., 2020). However, non-extractable polyphenols account for an important part of the total phenolic compounds in plant residues, and its extraction by alternative acid, alkali or enzymatic treatments would allow achieve much higher polyphenol yields (Dzah et al., 2020; Wang et al., 2020).
In addition to the extraction stage, another important aspect to take into account for further pharmaceutical and food applications, is the subsequent purification and characterization of pectic oligosaccharides and phenolic extracts obtained (Chiriac et al., 2021; Dranca & Oroian, 2018). Several alternatives have been evaluated for the purification of pectic oligosaccharides, including precipitation, membrane filtration, dialysis, ionic exchange, nitration, or combined methods (Dranca & Oroian, 2018; Míguez et al., 2016; Moreno-González & Ottens, 2021). Precipitation with ethanol achieves good pectin yields at low costs, while membrane filtration results in higher purities (Dranca & Oroian, 2018). Even though ethanol precipitation is the most commonly used method for the purification of pectin (Çilingir et al., 2021; Davis et al., 2021; Tsuru et al., 2021), only a few studies have evaluated the effect of the conditions on the recovery of oligosaccharides (Guo et al., 2016; Klinchongkon et al., 2019). Concerning the phenolic fraction, solid phase extraction and liquid–liquid extraction are between the most employed purification methods (Chiriac et al., 2021; Dzah et al., 2020; Moreno-González & Ottens, 2021). In this context, adsorption with macroporous resins is an efficient and easily scalable technology based on mass transfer, with low cost and simple design and operation (Niknam et al., 2021; Pérez-Armada et al., 2019). This approach has been previously successfully used for the purification and concentration of phenolic compounds from different extracts (Ismail et al., 2020; Ozgen & Sarıoglu, 2013; Pérez-Armada et al., 2019; Rodríguez-Juan et al., 2021; Saha et al., 2019). However, most works dealing with the recovery of phenolic compounds do not evaluate their purification (Davis et al., 2021; Kutlu et al., 2021; Moraes et al., 2020).
Several technologies have been tested for the valorization of melon by-products in the last years. In this line of research, recent studies deal with the recovery of pectin or pectic polysaccharides from melon peels using acid hydrolysis (Güzel & Akpınar, 2019; Muthukumaran et al., 2017; Raji et al., 2017), microwave-assisted extraction (Golbargi et al., 2021), autohydrolysis (Rico et al., 2020b), and extraction with deep eutectic solvents (DES) (Rico et al., 2021). Phenolic compounds have been also recovered from melon peels and seeds with organic solvents (Ganji et al., 2019; Mallek-Ayadi et al., 2018), in pressurized aqueous media (Rico et al., 2020b), with deep eutectic solvents (DES) (Rico et al., 2021) and by ultrasound-assisted extraction (Vella et al., 2019). On the other hand, the recovery of protein (Gómez-García et al., 2021a) and seed oil (Bouazzaoui et al., 2018) have also been addressed recently from melon by-products. To the best of our knowledge, the purification of phenolic compounds extracted from melon peels has not yet been studied. Moreover, pectin purification from melon peel extracts has only been assessed in several studies by precipitation in ethanol at fixed operational conditions, where concentrations between 50 and 75% were selected (Golbargi et al., 2021; Güzel & Akpınar, 2019; Muthukumaran et al., 2017; Raji et al., 2017; Rico et al., 2020b, 2021).
In this work, the purification of melon peel extracts obtained by two environmentally friendly methods (autohydrolysis and alkaline DES extraction) has been addressed. With this purpose, sequential stages of ethanol precipitation and resin adsorption/desorption were designed for the recovery of the pectic and phenolic fractions, respectively. A screening of the optimal conditions for the purification was carried out and the selected products were characterized.
Materials and Methods
Raw Material
Melon peels (var. piel de sapo), a by-product of fresh cut produce, were kindly supplied by FreshCut, S.L. (Vigo, Pontevedra, Spain). They were cut into small pieces and kept at −18 °C until use.
Preparation of Melon Peel Liquors
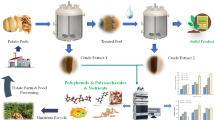
The two alternatives proposed for the valorization of melon peels are shown in Fig. 1 and explained in the following sections.
Aqueous Extraction
Melon peels (MP) were subjected to an aqueous extraction to separate soluble sugars, following the methodology previously reported by Rico et al. (2020a, 2021). Briefly, MP were first centrifuged at 2800 rpm for 15 min (SV4028, AEG Electrolux), separating the solids from the aqueous fraction. Next, the solid was washed for 15 min at room temperature with distilled water in a 10 L stirred reactor (40 g of water/g of oven dry MP) and solid and liquid fractions were separated again by centrifugation in the previously cited conditions. The washing and centrifugation steps were repeated once more.
Autohydrolysis
The hydrothermal treatment was carried out as described in a previous work (Rico et al., 2020b), with some modifications. Deionized water and wet water-insoluble solids (WIS) samples were mixed at the desired liquid to solid ratio (25 g water/g dry WIS) and heated in a 0.6 L stainless steel, pressurized reactor (model 4842 from Parr Instruments, Moline, IL, USA) up to 165 °C, and then cooled immediately. After separating by filtration, the liquid phase (AH) was stored at −18 °C until analyzed (following the methodology described below).
Solid/Liquid Extraction with Sodium Acetate/Urea Deep Eutectic Solvent
Sodium acetate/urea/water 1:3:1.6 (corresponding to a water content of 10% w/w) deep eutectic solvent (prepared by heating at 90 °C for 1 h) was used for solid/liquid extraction under experimental conditions previously optimized (Rico et al., 2021): 50 g DES/g dry WIS, 10 min at 90 °C. After the treatment, the solution was immediately poured in deionized water (2 mL water/g DES) to dissolve the extracted compounds and DES components. After separating by filtration, the liquid phase (DES-E) was stored at −18 °C and, in order to improve the oligosaccharides (OS) recovery, the solid phase was washed with deionized water three times for 15 min, using a liquid to solid ratio of 50 mL water/g dry WIS. The washing water of the three steps (DES-W), was recovered by filtration, mixed and stored at −18 °C. Aliquots of both phases were analyzed using the methodology described in the next sections.
Recovery of Pectic Oligosaccharides
The effect of final ethanol concentrations of 75, 80, and 85% v/v on the pectin recovery from AH has been assessed. The solutions were allowed to precipitate overnight at 4 °C, the precipitates were collected by centrifugation (for 20 min at 4000 rpm in a Rotixa 50 RS centrifuge, Hettich Lab Technology, Tuttlingen, Germany) and then washed twice with 96% ethanol. The obtained pectin was oven dried at 60 °C to a constant weight and ground. The same procedure was followed with DES-E and DES-W, using an ethanol concentration of 85% v/v. An aliquot of each precipitate was redissolved in water for further analysis (see the “Chemical Characterization of Liquors” section). The degree of acetylation (DA) was calculated as the molar ratio of acetic acid to galacturonic acid (determined by HPLC analysis), and the degree of methyl esterification (DM) was estimated from the FTIR spectra with the calibration curve determined by Manrique and Lajolo (2002).
where A1740 and A1630 are the areas under the peaks associated with esterified and free carboxyl groups. Homogalacturonan (HG) and Rhamnogalacturonan-I (RG-I) molar contents were calculated as defined by M’sakni et al. (2006), and the sugar ratios of each pectin were determined according to Houben et al. (2011):
where GalA, Rha, Gal, Ara, and Xyl are galacturonic acid, rhamnose, galactose, arabinose, and xylose contents in molar %. Sugar ratio 1 represents the linearity of pectin, sugar ratio 2 represents the contribution of RG to pectin population and sugar ratio 3 represents the branching of RG-I.
Recovery of Phenolic Compounds
The high content of ethanol in pectin-free liquors was evaporated under reduced pressure at 40 °C (using a rotatory evaporator R-210, Buchi, Switzerland), and then, the concentrated liquor was redissolved in water to reach similar concentrations to the original liquors (AH, DES-E, and DES-W). The recovery of phenolic compounds was evaluated with three commercial food grade non-ionic polymeric resins: Amberlite XAD4 (Sigma-Aldrich, Steinheim, Germany), XAD16HP (Supelco, Bellefonte, PA, USA) and XAD7HP (Sigma-Aldrich, Steinheim, Germany). Table 1 shows the main properties of these resins. Before the adsorption, resins were conditioned as follows: (1) washed twice with deionized water, at 30 °C and 200 rpm for 10 min; (2) activated with methanol during 30 min in the same conditions; and (3) rinsed with deionized water twice for 10 min to remove the methanol. Activated resins were kept at 4 °C until use.
Phenolic compounds adsorption from autohydrolysis pectin free liquors (AHL) was evaluated in an orbital shaker at 30 °C and 150 rpm for 120 min and at liquor to resin ratios of 2, 6, 10, and 20 mL/g, wet basis. Afterwards, the resins were separated by filtration and in order to complete the removal of undesired compounds, they were rinsed twice with deionized water for 20 min using a ratio of 3 mL water/g wet resin, while keeping the other operational variables as in the previous stage. Lastly, several desorption stages were performed in different media under the same experimental conditions. In the first set of experiments, two sequential desorption steps with ethanol 96% were evaluated. Afterwards, several desorption sequences were tested: (A) 50% ethanol, 96% ethanol and acidified 96% ethanol (pH 3); (B) 50% ethanol thrice; (C) 50% ethanol with 0.5% NaOH, 50% ethanol and acidified 50% ethanol (pH 3); and (D) acidified 50% ethanol (pH 3) thrice. The selected conditions for AHL were applied for DES extracts, testing both 96% and 50% ethanol for the desorption.
The total phenolic content (TPC) was determined for all liquid fractions after freeze drying and redissolving in a small volume of water (according to the methodology described below). The adsorption of TPC was calculated with the following equation:
where TPC0, TPCe, and TPCw are the total phenolic contents of the pectin free liquor before the adsorption, after the adsorption and of the washing water (in mg gallic acid equivalents (GAE)), respectively. The adsorption capacity of TPC was calculated as:
The desorption of TPC was calculated using:
where TPCD is the total phenolic content in the liquors after the desorption step (in mg GAE).
Finally, the total recovery of TPC was calculated as follows:
Analytical Methods
Analysis of the WIS
The dried WIS were analyzed according to the methodology previous reported by Rico et al. (2020b).
Chemical Characterization of Liquors
Aliquots of each liquor were filtered through 0.45 μm membranes and analyzed by HPLC for the determination of acids and monosaccharides, and subjected to enzymatic and acid posthydrolysis followed by HPLC analysis for the determination of oligosaccharides, as described by Rico et al. (2020a, 2021). In samples with high contents of DES compounds, OGalA (oligogalacturonides) were determined by the spectrophotometry method previously described (Rico et al., 2021). Acetic acid could not be determined in samples containing a high amount of sodium acetate since both compounds are eluted in the same peak. OS were expressed as monosaccharide (MS) equivalents. In autohydrolysis liquors, ash was determined by the T-244-om-93 method and protein was calculated from the nitrogen content (assuming 6.25 g protein/g nitrogen), which was determined by a modified Dumas method (Rico et al., 2020b). In the case of liquors containing urea, the protein was determined by a two-step method using the commercial Compat-Able™ Protein Assay Preparation Reagent Set (Thermo Scientific, USA) and BCA protein assay kit (Thermo Scientific, USA) (Nuutinen et al., 2019; Rico et al., 2021). The content of non-volatile compounds (NVC) was determined by oven-drying at 60 °C until constant weight. All samples were analyzed in triplicate.
TPC
The total phenolic content of samples was measured by the Folin–Ciocalteau method (Singleton & Rossi, 1965), and the results were expressed as mg of gallic acid equivalents (GAE)/g NVC or mg GAE/g dry WIS. All samples were analyzed in triplicate.
Antioxidant Capacity
Three different methods were used to assess the antioxidant capacity of the liquors: DPPH (α,α-Diphenyl-β-picrylhydrazyl radical scavenging assay), ABTS (2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid), and FRAP (ferric reducing antioxidant power) according to the methodology described by Gullón et al. (2017). The results were expressed as mg Trolox equivalents (TE)/g NVC as mean of three replicates.
Identification and Quantification of Major Phenolic Compounds Using HPLC–PDA-MS/MS
HPLC–PDA-MS/MS analysis was used to identify and quantify phenolic compounds using the methodology described in a previous work (Rico et al., 2020b). The separation was performed on a Phenomenex Luna C18 column (150 mm × 2 mm; 3 μm), with an injection volume of 5 μL and a flow of 300 μL/min. The mobile phase was 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in acetonitrile (B). The gradient used was: 98% A (v/v), 0–4 min; 98–80% A (v/v), 4–7 min; 80–10% A (v/v), 7–14 min; 10% A (v/v), 14–15 min; 10–98% A (v/v), 15–17 min. The mass spectrometer (AB SCIEX Triple Quad 3500 (AB Sciex, Foster City, CA)), equipped with a turbo V™ electrospray ionization source and to a Waters 996 photodiode array detector) was run in positive and negative ionization, using N2 as the nebulizer and collision gas, with an ion spray voltage of 4500 V, source temperature of 400 °C and nebulizer gas pressure of 55 psi.
Structural Characterization of the Recovered Pectic Oligosaccharides
Further information about structural characteristics of the recovered pectic oligosaccharides were obtained by FTIR and HPAEC-PAD analysis following the methodology of previous works (Gómez et al., 2013; Rico et al., 2020b, 2021).
The FTIR analysis was performed on a Nicolet 6700 Spectrometer. Samples of each pectin were assayed by the KBr pellet method. A total of 34 scans were accumulated per sample and the spectrum was obtained from a range of 4000–400 cm−1.
The HPAEC–-PAD analysis of the pectic oligosaccharides was carried out using an ICS3000 chromatographic system (Dionex, Sunnyvale, CA, USA), fitted with a CarboPac PA-1 column (2 mm i.d. × 250 mm) in combination with a CarboPac PA guard column (2 mm i.d. × 25 mm) and an ISC3000 PAD detector. A standard of pectic oligosaccharides was prepared by the hydrolysis of a 1% (w/w) solution of commercial polygalacturonic acid at 121 °C for 40 min at pH = 4.4 adjusted with NaOH, and commercial standards of GalA with DP 1, 2 and 3 were used as well to identify their peaks (not shown in the presented chromatogram).
Data Analysis
The HPLC and HPAEC-PAD chromatograms were processed using ChemStation LC B.04.03 software (Agilent Technologies). All data were analyzed using the commercial software Microsoft Excel (Microsoft, USA). The existence of significant differences among the results was analyzed by one-way analysis of variance (ANOVA), followed by t-test (two-sample, two-tailed distribution) using Microsoft Excel.
Results and Discussion
Chemical Composition of the WIS and the Obtained Liquors
MP were washed with water at room temperature to separate water soluble compounds. The aqueous extracts (E) accounted for 60.05 g/100 g MP and contained mainly glucose (22.21 g/100 g E), fructose (32.29 g/100 g E), and protein (10.61 g/100 g E). WIS represented the remaining 39.95 g/100 g MP and were mainly composed of glucan (24.76 g/100 g WIS), galacturonan (18.41 g/100 g WIS), Klason lignin (15.70 g/100 g WIS) and protein (9.70 g/100 g WIS).
The valorization of WIS has been approached through two strategies: autohydrolysis and sodium acetate/urea/water extraction (1:3:1.6), performed under improved operational conditions (see more details in the “Autohydrolysis” and “Solid/liquid extraction with sodium acetate/urea deep eutectic solvent” sections). In this case, a slightly acidic and an alkaline reaction media were selected in order to yield pectins with different chemical and structural properties. Table 2 shows the chemical composition of the liquors obtained from the two treatments proposed. OS were the main components of the liquors, with the highest amount being recovered by autohydrolysis (18.55 g OS/100 g WIS), while DES-W and DES-E liquors together accounted for 11.30 g OS/100 g WIS. As can been expected, the main OS detected was OGalA (with values close to 10 g/100 g WIS), followed by GalOS (galactose units in OS), AraOS (arabinose units in OS), Ac-OS (acetic acid units in OS), RhaOS (rhamnose units in OS), and minor contents of GOS, XOS, and ManOS (glucose, xylose, and mannose units in OS, respectively). The experimental conditions selected as well as the washing steps implemented in this study allowed to improve the OS recovery percentages obtained in our previous works (16.28 and 8.84 g/100 g WIS by autohydrolysis and DES extraction with sodium acetate/urea/water, respectively) (Rico et al., 2020b, 2021). A similar amount of monosaccharides was solubilized by each treatment (around 2 g/100 g WIS), which were mostly glucose and fructose. Some organic acids were also found in the liquors (formic, glucuronic, acetic and citric acids), especially in AH with 2.19 g/100 g WIS. Concerning the TPC, its value was higher for AH liquor (10.38 mg GAE/g WIS), followed by DES-E and DES-W (4.00 and 2.12 mg GAE/g WIS, respectively).
Recovery of Pectic Oligosaccharides
For the recovery of pectin by precipitation in alcohol media, three ethanol concentrations were tested: 75, 80 and 85% (v/v). This interval was selected not only based on our preliminary results (Rico et al., 2020b, 2021), but also on Guo and coworkers’ ones, who reported that high ethanol contents could favor the precipitation of highly branched pectin fractions (Guo et al., 2016). Moreover, Klinchongkon and coworkes reported a significant increase in carbohydrates precipitation at ethanol concentrations higher than 80% (Klinchongkon et al., 2019). The effect of ethanol concentration on the recovery of the main pectic oligosaccharides and protein in AH is shown in Table 3. From the results, it should be noted that an increase in ethanol content from 75 to 85% resulted in an increase in the total OS recovery of 8%. This way, the total oligosaccharides content was close to 72%, considerably higher than that in autohydrolysis liquors (62%). Therefore, the effectiveness of this method for the purification of pectic oligosaccharides is proven. Besides, the total amount of precipitate increased progressively when increasing the concentration of ethanol from 75 to 85% (12.32 to 16.11 g/100 g WIS). These results are in contrast with those reported by Guo et al. (2016) for pectic oligosaccharides, where no improvement was found when increasing the ethanol concentration over 78%. However, they are in agreement with the results reported by Klinchongkon et al. (2019) for mannooligosaccharides. This information confirms the initial hypothesis of higher ethanol concentrations favoring the precipitation of neutral oligosaccharides. Thus, in this work, the highest oligosaccharide recoveries were found using the highest ethanol concentration, with OGalA and GalOS recoveries of 71.26 and 75.79%, respectively.
In these conditions, the pectin yields for AH, DES-E, and DES-W were 16.11, 5.20, and 12.85 g/100 g WIS, respectively. These values are in range with data given in literature for the extraction of pectin from melon by-products with different technologies (Güzel & Akpınar, 2019; Kazemi et al., 2021; Muthukumaran et al., 2017; Rico et al., 2020b, 2021). The amount of pectin recovered from the DES treatment (DES-EP + DES-WP) is higher than that of autohydrolysis (AHP), but the total amount of OS in those pectins is lower (11.55 g OS/100 g WIS for AHP and 10.68 g OS/100 g WIS for DES-EP + DES-WP). AHP presented an OS content of 71.67%, while DES-EP and DES-WP a value slightly under 60%, due to a lower presence of neutral oligosaccharides, mainly GalOS. As can be seen in Table 4, the three pectins contained around 45% of galacturonic acid, close to those reported for melon by-products in other works (47–55%) (Muthukumaran et al., 2017; Rico et al., 2020b, 2021). After galacturonic acid, the next main compound found in pectin was galactose, with contents of 17.82% in AHP and 8.17 and 7.29% in DES-EP and DES-WP, respectively.
Recently, there is an increasing interest in targeted extraction of RG-I due to its superior bioactivities over HG, including prebiotic potential, and immunomodulatory and anti-apoptotic activities (Hou et al., 2022; Mao et al., 2019a; Wu et al., 2020; Zhu et al., 2019). The alkaline DES treatment proposed in this study resulted in pectins with higher HG and lower RG-I than autohydrolysis ones (see Table 4). However, other authors reported that alkaline conditions tend to extract RG-I-rich pectins, while neutral and acid ones result in highly linear pectins (Mao et al., 2019a, b). Moreover, AHP showed a higher amount of neutral sugar side chains than DES-EP and DES-WP (with RG-I contents of 38.90, 24.62, and 23.72%, respectively), which a priori was not expected due to the alkaline nature of the DES used. However, this can be explained by the temperature selected for the autohydrolysis treatment, since it was chosen to maximize the amount of total OS. OGalA has a higher susceptibility to autohydrolysis reactions than neutral OS (Gómez et al., 2013; Martínez et al., 2009, 2010; Rico et al., 2020b), therefore increasing the treatment temperature could degrade HG and thus increase the content in neutral OS. In this line, recent studies also observed that acid extraction and hydrothermal treatment can be tuned to recover pectins with different compositions (Chen et al., 2021; Zhang et al., 2022). The RG-I contents and sugar ratios obtained for the pectins recovered in this work (see Table 4) are in range with those of sugar beet pulp pectins extracted in alkaline media (Sugar ratio 1: 0.9–4.0, sugar ratio 2: 0.01–0.08, sugar ratio 3: 11.1–16.2) (Mao et al., 2019b).
All pectins were low methoxyl, with DES-WP having the lowest DM (8.85%) and AHP the highest (27.57%). This is in agreement with the dominant degradation mechanisms of pectin at pH 3–7 (β-elimination) and neutral to alkaline (demethoxylation), which are accelerated at high temperatures such as the one used for autohydrolysis in this work (Mao et al., 2019b). AHP also had the highest degree of acetylation (16.53%). Besides oligosaccharides, pectins contained a small amount protein (under 5% in every case), organic acids, mainly citric and glucuronic (1–3%), phenolic compounds (4–7 mg GAE/g pectin) and negligible contents of monosaccharides (≤ 0.03%).
Structural Characterization
FTIR and HPAEC-PAD were used to acquire information about the structure of the three pectins recovered in this work.
The FTIR spectra (Fig. 2) confirmed the presence of the characteristic functional groups of pectin, with OH and CH stretching vibrations at 3271–3345 cm−1 and 2935–2940 cm−1, and esterified and free carboxylic acid vibrations at 1732–1738 cm−1 and 1596–1604 cm−1 (Chen & Lahaye, 2021; Chen et al., 2021; Kazemi et al., 2021; Manrique & Lajolo, 2002; Rico et al., 2021). The bands of esterified carboxyl groups (1732–1738 cm−1) had lower intensity for pectins from the DES treatment, especially DES-WP, due to the demethoxylation at alkaline conditions, resulting in lower DM (Hou et al., 2021). The peak at 1650–1651 cm−1 in DES-EP and DES-WP could be related to a small amount of protein (Kpodo et al., 2017). The signals at around 1410, 1370, and 1330 cm−1 might be related to symmetric stretching of free carboxyl groups, CH stretching vibrations and the COO functional group in pectin, respectively (Gnanasambandam & Proctor, 2000; Kpodo et al., 2017; Muthukumaran et al., 2017; Rico et al., 2020b). Finally, the signals between 900 and 1200 cm−1 are part of the “finger print” area (unique to a compound and difficult to interpret), and could be attributed to the stretching of glycosidic bonds in the pectin structure (Gnanasambandam & Proctor, 2000; Hou et al., 2021; Kazemi et al., 2021; Raji et al., 2017).
Figure 3 shows the HPAEC-PAD chromatograms obtained for the recovered pectins and hydrolyzed commercial polygalacturonic acid (PGA), as reference. Commercial galacturonic acid standards with DP1, DP2, and DP3 (degree of polymerization 1, 2, and 3) were also used to identify the corresponding peaks. Linear OGalA are eluted according to their polymerization degree, since the methyl ester groups are removed during the analysis due to the high pH of the eluent (Gómez et al., 2013; Ralet et al., 2005). The three pectins showed a similar DP distribution, from DP2 to over 20. The presence of linear OGalA was confirmed by the peaks aligning with the standards in each pectin, with some of these peaks being more notably present in AHP than in DES-EP and DES-WP (especially DP7, DP8, and DP9). However, an important part of the peaks shown in the chromatogram do not correspond to the linear OGalA shown by the standard, which means they could be attributed to the presence of branched OGalA or neutral OS. This is in agreement with the considerable amount of RG estimated previously for each pectin. The response areas of DES-EP for high DP were higher than for DES-WP and AHP, indicating a higher concentration of high DP oligomers. This observation suggests that the autohydrolysis treatment caused more depolymerization than the DES treatment.
Recovery of Phenolic Compounds
The liquid phase from pectin precipitation was subjected to rotatory evaporation until concentrated to the original volume to eliminate the ethanol. These liquors (AHL for autohydrolysis, DES-EL for the DES extracts and DES-WL for the aqueous washing after DES extraction) contained about 90% of the TPC of the liquors before precipitation. In order to recover and concentrate the phenolic compounds of these liquors, adsorption stages with Amberlite XAD4, XAD16HP, and XAD7HP were proposed. They were selected based on previous literature focused on the recovery of phenolic compounds from aqueous and DES solutions (García et al., 2016; Niknam et al., 2021; Pérez-Armada et al., 2019; Rodríguez-Juan et al., 2021; Saha et al., 2019).
Adsorption
To optimize the phenolics adsorption from pectin free AH liquors, liquid/resin ratios (mL/g, wet basis) of 2, 6, 10, and 20 were firstly selected based on the bibliography (Ferri et al., 2011; Pérez-Armada et al., 2019; Yang et al., 2016). As can be seen in Table 5, in these experiments, an increase in the liquid/resin ratio resulted in higher adsorption capacities, with values in the range 0.37–2.29 mg GAE/g wet resin. Previous studies found a lot of variability in the capacities of these resins, which could be due to the different phenolic profiles with varying molecular size and form (Silva et al., 2007). For instance, Soto et al. (2012) using the same resins at a ratio of 1.7 mL/g with winery wastes reported capacities around 2 mg GAE/g wet resin, whereas Ismail et al. (2020) applying XAD16 to baobab pulp at a ratio of 50 mL/g found values under 2 mg GAE/g. On the other hand, Pérez-Armada et al. (2019) evaluated liquid/resin ratios from 1.7 to 500 mL/g dry resin and found capacities between 1 and 95 mg GAE/g dry resin for XAD16HP with hazelnut shell autohydrolysis liquors.
Regarding the adsorption yields, increasing the amount of resin (decreasing the liquid/resin ratio) increased the adsorption rate significantly for every resin in this work. However, Niknam et al. (2021), in a study performed with olive pomace extracts, found small differences in the adsorption of phenolic compounds when increasing the liquid/resin ratio from 10 to 20 using XAD4, XAD16, and NPAC resins. And, in the above cited work, Pérez-Armada et al. (2019) found virtually no decrease in adsorption with XAD4, SP700, and SP825 resins when increasing the liquid/resin ratio from 1.7 to 5; however, XAD16HP resin did decrease its adsorption from around 80% to around 40%.
Comparing the three studied resins, the highest adsorption capacity was obtained with XAD16HP, followed by XAD4 and XAD7HP. This could be due to the higher specific surface area and pore volume of XAD16HP (Pérez-Armada et al., 2019). The decrease in capacity when increasing resin dosage was lower for XAD4 than XAD16HP, with both resins achieving similar adsorption yields with the highest resin dosage (liquid/resin ratio of 2). However, XAD7HP achieved lower yields in every case. The same trend was found for the adsorption of antioxidants from winery wastes (Soto et al., 2012), where XAD16HP and XAD4 resulted in adsorptions of around 80% whereas XAD7HP only reached values of 70%. XAD4 and XAD16HP basically differ on pore diameter dimensions. Therefore, XAD16HP is more suited for the recovery of larger molecules, but their adsorption performances are similar for small compounds (Ferri et al., 2011). This fact could explain the better results obtained with XAD16 in comparison with XAD4 in previous studies (Niknam et al., 2021; Pérez-Armada et al., 2019).
In order to evaluate the effect of the ethanol presence and try to improve the adsorption rates, an additional rotatory evaporation stage was applied to the pectin free liquors to eliminate most of the liquid. Afterwards, deionized water was added up to the original volume to maintain the concentration of phenolic compounds (0.42 g GAE/L). This way, adsorption yield was increased for every resin by an average of 9%.
Considering the results of the adsorption tests, XAD4 and XAD16HP and the liquid/resin ratio of 2 were selected for the following experiments.
Desorption
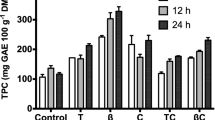
Firstly, desorption in two steps with 96% ethanol was assayed, achieving desorption percentages of only around 50% with XAD4 and XAD16HP. To improve this results, four sequences of three desorption steps were tested with both resins: (A) 50% ethanol, 96% ethanol, acidified 96% ethanol (pH 3), (B) 50% ethanol thrice, (C) 50% ethanol with 0.5% NaOH, 50% ethanol, acidified 50% ethanol (pH 3), (D) acidified 50% ethanol (pH 3) thrice. The desorption yields achieved in these experiments are shown in Fig. 4.
The sequential desorption with pH changes (denoted as C) resulted in poor desorption yields, similar to the ones achieved with 96% ethanol. No difference was detected in the first step performed at basic pH. 50% ethanol showed higher desorption rates than 96% ethanol, with values of 72.69 and 66.14% in two steps for XAD4 and XAD16HP, respectively. Besides, when using 50% ethanol in the first step and 96% in the second one, the results were also lower than when using 50% ethanol twice (66.83 and 61.75% for XAD4 and XAD16HP, respectively). These results are in agreement with those reported by Yang et al. (2016) with XAD16 resin, during the desorption of adlay bran free phenolics with ethanol.
As can be seen in Fig. 4, acidifying the solvent in the first and fourth sequences had a slight positive effect. However, that difference was not significant, so 50% ethanol in three steps was selected as the optimal desorption method. The desorption yields in these conditions were 76.58% and 75.29% for XAD4 and XAD16HP, respectively. These values compare favorably with the reported yields for winery wastes with HP20, SP207, and XAD16HP resins (51–67%) (Soto et al., 2012). However, they are in range with the ones obtained in a recent study performed with olive pomace extract using XAD4 and XAD16 with a liquid/resin ratio of 10 mL/g (80.08 and 70.30%, respectively), but are lower than those achieved with a ratio of 20 mL/g (96.99 and 79.89%, respectively) (Niknam et al., 2021).
Considering there was no significant difference between the desorption yield of XAD4 and XAD16HP, XAD4 was chosen as the best option since it achieved a slightly higher adsorption yield than XAD16HP. Therefore, the selected purification sequence for authohydrolysis pectin free liquors was the following: XAD4 resin, liquid/resin 2 and 3 desorption steps with 50% ethanol. Under these operational conditions, a 66.56% of the TPC is adsorbed into the resin, of which 76.85% is desorbed (AHRE stream). In this way, a 50.97% of the TPC present in the pectin free liquor (AHL) would be recovered, which would be equivalent to 46.83% of the TPC contained in the autohydrolysis liquor (total recovered TPC of 4.86 mg GAE/g WIS). Similar TPC recoveries were achieved for olive pomace extracts (55.86%) (Niknam et al., 2021) and for a stock solution of low molecular weight phenols (Ferri et al., 2011), using in both studies XAD16 resin and 50% ethanol as desorbing solvent.
The selected conditions were applied as well to the liquors obtained from DES extraction: DES-EL, DES-WL, and a mixture of both (DES-L). The initial concentrations of phenolic compounds in DES-EL, DES-WL, and DES-L were 0.026, 0.018, and 0.024 g GAE/L, respectively. The adsorption of TPC for DES-EL (38.70%) and DES-L (37.79%) was similar, whereas for DES-WL, it was slightly higher (41.55%). These results suggest that, even if highly diluted, the DES lowers considerably the adsorption of phenolic compounds into the XAD4 resin. However, Panić et al. (2019) reported an increase in the recovery of anthocyanins using Sepabeads SP207 after diluting the DES to 80% water.
As for the desorption, in this case, 50% and 96% ethanol were tested. The desorption yields obtained with three liquors were similar, with 50% ethanol giving the best results (73.29, 78.75, and 76.88% for DES-EL, DES-WL, and DES-L, respectively). Hence, in this work, 50% ethanol was selected as desorption solvent for the recovery of phenolic compounds from XAD4 in all the liquors tested. This way, the final recoveries of TPC for each of the DES liquors were 28.37% for DES-ERE (recovered from DES-EL), 32.72% for DES-WRE (from DES-WL) and 29.05% for DES-RE (from DES-L). In brief, 37.79% of the TPC is adsorbed into the resin, of which 76.88% is desorbed (DES-RE stream); achieving a recovery of 29.05% of the TPC present in the pectin free liquor (DES-L) or 23.66% of the TPC present in both DES-E and DES-W liquors (total recovered TPC of 1.45 mg GAE/g WIS).
Antioxidant Capacity and Phenolic Content of the Purified Streams
The TPC and antioxidant capacities (determined by DPPH, ABTS, and FRAP methods) of the obtained fractions are summarized in Table 6. It should be noted that the recovered phenolic compounds showed an improved antioxidant capacity. For autohydrolysis streams, AHRE contained 46.83% of the TPC of the original liquor (AH), but about 60% of the antioxidant capacities (measured by the three methods). When referring the results to dry mass content, antioxidant capacities are about 200% higher for the purified stream than the original liquor, while the TPC content is only 127% higher (79.55 mg GAE/g NVC in AHRE versus 35.05 mg GAE/g NVC in AH), showing the improved antioxidant capacity. This TPC yield is in range with those obtained for hazelnut shell autohydrolysis liquors using XAD4 and XAD16HP (110 and 81 mg GAE/g NVC, respectively) (Pérez-Armada et al., 2019).
Comparing the results with a previous autohydrolysis work, the purified phenolic compounds contained a higher amount of TPC (4.86 versus 1.89 mg GAE/g WIS) than the unpurified liquors from a different batch of melon peels using autohydrolysis at 140 °C (Rico et al., 2020b). Moreover, they presented higher DPPH and FRAP capacities (1.41 and 1.99 versus 0.65 and 0.83 mg TE/g WIS) but lower ABTS antioxidant capacity (3.04 versus 7.52 mg TE/g WIS). Therefore, the different autohydrolysis approach taken in this work has improved the recovery of phenolic compounds with shown antioxidant capacity while also producing a similar amount of pectic oligosaccharides.
Regarding the DES treatment, DES-RE contained 23.66% of the TPC of the original liquors (DES-E and DES-W) and about 40% of the capacities measured by DPPH and FRAP, but only 18.51% of the capacity measured by ABTS. The ABTS capacity could be overestimated by the effect of the DES on the kinetics of free radical scavenging reactions in the assay, as hypothesized previously (Ozturk et al., 2018; Rico et al., 2021). The lower content of DES components after purification by resin adsorption would lower this effect and give a more reliable result. The TPC yields were much lower than the ones achieved for AHRE: 2.58, 12.55, and 4.08 mg GAE/g NVC for streams DES-ERE, DES-WRE, and DES-RE, respectively, suggesting that the DES components are still present. The same can be said for the antioxidant capacities, with the capacities of AHRE being about 20 times higher than those of DES-RE.
In order to evaluate the composition of the phenolic compounds, the four streams were also analyzed by HPLC–PDA-MS/MS. As shown in Table 6, two phenolic acids (protocatechuic and ferulic acids) and three flavonoids (orientin or luteolin-8-glucoside, vitexin or apigenin 8-glucoside, and naringenin) were detected in all streams. Protocatechuic and ferulic acids and naringenin have been previously found in melon peel extracts in other works (Gómez-García et al., 2021b; Mallek-Ayadi et al., 2017; Vella et al., 2019), whereas orientin and vitexin have only been detected in melon fruits (Filho et al., 2020). In agreement with the TPC, all the phenolic compounds were found in higher amounts in AHRE, where the high content of naringenin (199.51 µg/g NVC) stands out, followed by protocatechuic acid (20.79 µg/g NVC). However, there was not much of a difference in the content of ferulic acid and vitexin between AHRE and DES-RE. The differences found in the phenolic profile of the purified extracts obtained by each treatment could explain the discrepancies observed in adsorption yields, as stated in the previous section.
Conclusions
The sequential recovery of pectic oligosaccharides and phenolic compounds from melon peel extracts can be achieved by ethanol precipitation followed by resin adsorption. The precipitation with 85% ethanol managed to recover pectins with a galacturonic acid content of about 45% from autohydrolysis liquors and DES extracts. The presence of low methoxyl GalA was confirmed by FTIR and both linear and branched OGalA with DP from 2 to 20 were confirmed by HPAEC–PAD for every pectin. Resin adsorption with XAD4 followed by desorption with 50% ethanol achieved recoveries of 50.97 and 29.05% of the total phenolic content for the pectin free liquors, with final contents of 79.55 and 4.08 mg GAE/g NVC, for autohydrolysis and DES treatments, respectively. Phenolic acids (protocatechuic and ferulic acids) and flavonoids (orientin, vitexin and naringenin) were detected by HPLC–PDA-MS/MS in every case. These results prove that both the oligosaccharide and phenolic fraction present in the extracts can be recovered using ethanol precipitation and resin adsorption as a sequential purification scheme.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Ac-OS:
-
Acetic acid units in oligosaccharides
- AH:
-
Autohydrolysis liquor
- AHL:
-
Liquor from autohydrolysis after pectin precipitation
- AHP:
-
Pectin from autohydrolysis liquor
- AHRE:
-
Phenolic fraction from autohydrolysis recovered with resin adsorption
- AHRL:
-
Autohydrolysis liquor free from pectin and phenolics
- AHS:
-
Solid from autohydrolysis treatment
- Ara:
-
Arabinose
- AraOS:
-
Arabinose units in oligosaccharides
- DA:
-
Degree of acetylation
- DES:
-
Deep eutectic solvent
- DES-E:
-
Liquor from DES extraction
- DES-EL:
-
Liquor from DES extraction after pectin precipitation
- DES-EP:
-
Pectin from DES extracts
- DES-ERE:
-
Phenolic fraction recovered with resin adsorption from DES-EL
- DES-L:
-
Mixture of DES-EL and DES-WL
- DES-RE:
-
Phenolic fraction recovered with resin adsorption from DES-L
- DES-RL:
-
DES extracts free from pectin and phenolics
- DESS:
-
Solid from DES treatment
- DES-W:
-
Liquor from water washing after DES extraction
- DES-WL:
-
Liquor from water washing after DES extraction and pectin precipitation
- DES-WP:
-
Pectin from water washing after DES treatment
- DES-WRE:
-
Phenolic fraction recovered with resin adsorption from DES-WL
- DM:
-
Degree of methyl esterification
- DP:
-
Degree of polymerization
- E:
-
Water-soluble extractives
- GAE:
-
Gallic acid equivalents
- Gal:
-
Galactose
- GalA:
-
Galacturonic acid
- GalOS:
-
Galactose units in oligosaccharides
- GOS:
-
Glucose units in oligosaccharides
- HG:
-
Homogalacturonan
- ManOS:
-
Mannose units in oligosaccharides
- MP:
-
Melon peels
- MS:
-
Monosaccharides
- NVC:
-
Non-volatile content
- OGalA:
-
Oligogalacturonides
- OS:
-
Oligosaccharides
- PGA:
-
Polygalacturonic acid
- PS-DVB:
-
Polystyrene-divinylbenzene
- RG:
-
Rhamnogalacturonan
- Rha:
-
Rhamnose
- RhaOS:
-
Rhamnose units in oligosaccharides
- TE:
-
Trolox equivalents
- TPC:
-
Total phenolic content
- WIS:
-
Water-insoluble solids
- XOS:
-
Xylose units in oligosaccharides
- Xyl:
-
Xylose
References
Bouazzaoui, N., Bouajila, J., Camy, S., Mulengi, J. K., & Condoret, J. -S. (2018). Fatty acid composition, cytotoxicity and anti-inflammatory evaluation of melon (Cucumis melo L. Inodorus) seed oil extracted by supercritical carbon dioxide. Separation Science and Technology, 53(16), 2622–2627. https://doi.org/10.1080/01496395.2018.1464579
Chen, J., Cheng, H., Zhi, Z., Zhang, H., Linhardt, R. J., Zhang, F., Chen, S., & Ye, X. (2021). Extraction temperature is a decisive factor for the properties of pectin. Food Hydrocolloids, 112, 106160. https://doi.org/10.1016/j.foodhyd.2020.106160
Chen, M., & Lahaye, M. (2021). Natural deep eutectic solvents pretreatment as an aid for pectin extraction from apple pomace. Food Hydrocolloids, 115, 106601. https://doi.org/10.1016/j.foodhyd.2021.106601
Chiriac, E., Chiţescu, C., Geană, E. -I., Gird, C., Socoteanu, R., & Boscencu, R. (2021). Advanced analytical approaches for the analysis of polyphenols in plants matrices–a review. Separations, 8(5), 65. https://doi.org/10.3390/separations8050065
Çilingir, S., Goksu, A., & Sabanci, S. (2021). Production of pectin from lemon peel powder using ohmic heating-assisted extraction process. Food and Bioprocess Technology, 14(7), 1349–1360. https://doi.org/10.1007/s11947-021-02636-9
Davis, E. J., Spadoni Andreani, E., & Karboune, S. (2021). Production of extracts composed of pectic oligo/polysaccharides and polyphenolic compounds from cranberry pomace by microwave-assisted extraction process. Food and Bioprocess Technology, 14(4), 634–649. https://doi.org/10.1007/s11947-021-02593-3
Ding, Y., Morozova, K., Scampicchio, M., & Ferrentino, G. (2020). Non-extractable polyphenols from food by-products: Current knowledge on recovery, characterisation, and potential applications. Processes, 8(8), 925. https://doi.org/10.3390/pr8080925
Dranca, F., & Oroian, M. (2018). Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Research International, 113, 327–350. https://doi.org/10.1016/j.foodres.2018.06.065
Dzah, C. S., Duan, Y., Zhang, H., Serwah Boateng, N. A., & Ma, H. (2020). Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends in Food Science & Technology, 99, 375–388. https://doi.org/10.1016/j.tifs.2020.03.003
FAO. (2021). Faostat Database. www.fao.org/faostat. Accessed 13 Dec 2021
Ferri, F., Bertin, L., Scoma, A., Marchetti, L., & Fava, F. (2011). Recovery of low molecular weight phenols through solid-phase extraction. Chemical Engineering Journal, 166(3), 994–1001. https://doi.org/10.1016/j.cej.2010.11.090
Filho, F. O., Silva, E. D. O., Lopes, M. M. D. A., Ribeiro, P. R. V., Oster, A. H., Guedes, J. A. C., Zampieri, D. D. S., Bordallo, P. D. N., & Zocolo, G. J. (2020). Effect of pulsed light on postharvest disease control-related metabolomic variation in melon (Cucumis melo) artificially inoculated with Fusarium pallidoroseum. PLoS ONE, 15(4), e0220097. https://doi.org/10.1371/journal.pone.0220097
Ganji, S. M., Singh, H., & Friedman, M. (2019). Phenolic content and antioxidant activity of extracts of 12 melon (Cucumis melo) peel powders prepared from commercial melons. Journal of Food Science, 84(7), 1943–1948. https://doi.org/10.1111/1750-3841.14666
García, A., Rodríguez-Juan, E., Rodríguez-Gutiérrez, G., Rios, J. J., & Fernández-Bolaños, J. (2016). Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chemistry, 197, 554–561. https://doi.org/10.1016/j.foodchem.2015.10.131
Gnanasambandam, R., & Proctor, A. (2000). Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chemistry, 68(3), 327–332. https://doi.org/10.1016/S0308-8146(99)00191-0
Golbargi, F., Gharibzahedi, S. M. T., Zoghi, A., Mohammadi, M., & Hashemifesharaki, R. (2021). Microwave-assisted extraction of arabinan-rich pectic polysaccharides from melon peels: Optimization, purification, bioactivity, and techno-functionality. Carbohydrate Polymers, 256, 117522. https://doi.org/10.1016/j.carbpol.2020.117522
Gómez-García, R., Campos, D. A., Aguilar, C. N., Madureira, A. R., & Pintado, M. (2021a). Biological protein precipitation: A green process for the extraction of cucumisin from melon (Cucumis melo L. inodorus) by-products. Food Hydrocolloids, 116, 106650. https://doi.org/10.1016/j.foodhyd.2021.106650
Gómez-García, R., Campos, D. A., Oliveira, A., Aguilar, C. N., Madureira, A. R., & Pintado, M. (2021b). A chemical valorisation of melon peels towards functional food ingredients: Bioactives profile and antioxidant properties. Food Chemistry, 335, 127579. https://doi.org/10.1016/j.foodchem.2020.127579
Gómez, B., Gullón, B., Yáñez, R., Parajó, J. C., & Alonso, J. L. (2013). Pectic oligosacharides from lemon peel wastes: Production, purification, and chemical characterization. Journal of Agricultural and Food Chemistry, 61(42), 10043–10053. https://doi.org/10.1021/jf402559p
Gullón, B., Eibes, G., Moreira, M. T., Dávila, I., Labidi, J., & Gullón, P. (2017). Antioxidant and antimicrobial activities of extracts obtained from the refining of autohydrolysis liquors of vine shoots. Industrial Crops and Products, 107, 105–113. https://doi.org/10.1016/j.indcrop.2017.05.034
Guo, X., Meng, H., Zhu, S., Tang, Q., Pan, R., & Yu, S. (2016). Stepwise ethanolic precipitation of sugar beet pectins from the acidic extract. Carbohydrate Polymers, 136, 316–321. https://doi.org/10.1016/j.carbpol.2015.09.003
Güzel, M., & Akpınar, Ö. (2019). Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food and Bioproducts Processing, 115, 126–133. https://doi.org/10.1016/j.fbp.2019.03.009
Hou, Z., Chen, S., & Ye, X. (2021). High pressure processing accelarated the release of RG-I pectic polysaccharides from citrus peel. Carbohydrate Polymers, 263, 118005. https://doi.org/10.1016/j.carbpol.2021.118005
Hou, Z., Hu, X., Luan, L., Yu, C., Wang, X., Chen, S., & Ye, X. (2022). Prebiotic potential of RG-I pectic polysaccharides from Citrus subcompressa by novel extraction methods. Food Hydrocolloids, 124, 107213. https://doi.org/10.1016/j.foodhyd.2021.107213
Houben, K., Jolie, R. P., Fraeye, I., Van Loey, A. M., & Hendrickx, M. E. (2011). Comparative study of the cell wall composition of broccoli, carrot, and tomato: Structural characterization of the extractable pectins and hemicelluloses. Carbohydrate Research, 346(9), 1105–1111. https://doi.org/10.1016/j.carres.2011.04.014
Huang, J., Hu, Z., Hu, L., Li, G., Yao, Q., & Hu, Y. (2021). Pectin-based active packaging: A critical review on preparation, physical properties and novel application in food preservation. Trends in Food Science & Technology, 118, 167–178. https://doi.org/10.1016/j.tifs.2021.09.026
Ismail, B. B., Yusuf, H. L., Pu, Y., Zhao, H., Guo, M., & Liu, D. (2020). Ultrasound-assisted adsorption/desorption for the enrichment and purification of flavonoids from baobab (Adansonia digitata) fruit pulp. Ultrasonics Sonochemistry, 65, 104980. https://doi.org/10.1016/j.ultsonch.2020.104980
Kazemi, M., Amiri Samani, S., Ezzati, S., Khodaiyan, F., Hosseini, S. S., & Jafari, M. (2021). High-quality pectin from cantaloupe waste: Eco-friendly extraction process, optimization, characterization and bioactivity measurements. Journal of the Science of Food and Agriculture, 101(15), 6552–6562. https://doi.org/10.1002/jsfa.11327
Klinchongkon, K., Bunyakiat, T., Khuwijitjaru, P., & Adachi, S. (2019). Ethanol Precipitation of Mannooligosaccharides from Subcritical Water-Treated Coconut Meal Hydrolysate. Food and Bioprocess Technology, 12(7), 1197–1204. https://doi.org/10.1007/s11947-019-02288-w
Kpodo, F. M., Agbenorhevi, J. K., Alba, K., Bingham, R. J., Oduro, I. N., Morris, G. A., & Kontogiorgos, V. (2017). Pectin isolation and characterization from six okra genotypes. Food Hydrocolloids, 72, 323–330. https://doi.org/10.1016/j.foodhyd.2017.06.014
Kumar, M., Tomar, M., Saurabh, V., Mahajan, T., Punia, S., Contreras, M. D. M., Rudra, S. G., Kaur, C., & Kennedy, J. F. (2020). Emerging trends in pectin extraction and its anti-microbial functionalization using natural bioactives for application in food packaging. Trends in Food Science and Technology, 105, 223–237. https://doi.org/10.1016/j.tifs.2020.09.009
Kutlu, N., Isci, A., Sakiyan, O., & Yilmaz, A. E. (2021). Extraction of phenolic compounds from cornelian cherry (Cornus mas L.) using microwave and ohmic heating assisted microwave methods. Food and Bioprocess Technology, 14(4), 650–664. https://doi.org/10.1007/s11947-021-02588-0
Li, D., Li, J., Dong, H., Li, X., Zhang, J., Ramaswamy, S., & Xu, F. (2021). Pectin in biomedical and drug delivery applications: A review. International Journal of Biological Macromolecules, 185, 49–65. https://doi.org/10.1016/j.ijbiomac.2021.06.088
M’sakni, N. H., Majdoub, H., Roudesli, S., Picton, L., Le Cerf, D., Rihouey, C., & Morvan, C. (2006). Composition, structure and solution properties of polysaccharides extracted from leaves of Mesembryanthenum crystallinum. European Polymer Journal, 42(4), 786–795. https://doi.org/10.1016/j.eurpolymj.2005.09.014
Mallek-Ayadi, S., Bahloul, N., & Kechaou, N. (2017). Characterization, phenolic compounds and functional properties of Cucumis melo L. peels. Food Chemistry, 221, 1691–1697. https://doi.org/10.1016/j.foodchem.2016.10.117
Mallek-Ayadi, S., Bahloul, N., & Kechaou, N. (2018). Chemical composition and bioactive compounds of Cucumis melo L. seeds : Potential source for new trends of plant oils. Process Safety and Environmental Protection, 113, 68–77. https://doi.org/10.1016/j.psep.2017.09.016
Manrique, G. D., & Lajolo, F. M. (2002). FT-IR spectroscopy as a tool for measuring degree of methyl esterification in pectins isolated from ripening papaya fruit. Postharvest Biology and Technology, 25(1), 99–107. https://doi.org/10.1016/S0925-5214(01)00160-0
Mao, G., Wu, D., Wei, C., Tao, W., Ye, X., Linhardt, R. J., Orfila, C., & Chen, S. (2019a). Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends in Food Science and Technology, 94, 65–78. https://doi.org/10.1016/j.tifs.2019.11.001
Mao, Y., Lei, R., Ryan, J., Arrutia Rodriguez, F., Rastall, B., Chatzifragkou, A., Winkworth-Smith, C., Harding, S. E., Ibbett, R., & Binner, E. (2019b). Understanding the influence of processing conditions on the extraction of rhamnogalacturonan-I “hairy” pectin from sugar beet pulp. Food Chemistry: X, 2, 100026. https://doi.org/10.1016/j.fochx.2019.100026
Martínez, M., Gullón, B., Schols, H. A., Alonso, J. L., & Parajó, J. C. (2009). Assessment of the production of oligomeric compounds from sugar beet pulp. Industrial & Engineering Chemistry Research, 48(10), 4681–4687. https://doi.org/10.1021/ie8017753
Martínez, M., Yáñez, R., Alonso, J. L., & Parajó, J. C. (2010). Chemical production of pectic oligosaccharides from orange peel wastes. Industrial & Engineering Chemistry Research, 49(18), 8470–8476. https://doi.org/10.1021/ie101066m
Míguez, B., Gómez, B., Gullón, P., Gullón, B., & Alonso, J. L. (2016). Pectic oligosaccharides and other emerging prebiotics. Probiotics and Prebiotics in Human Nutrition and Health (pp. 267–322). InTech. https://doi.org/10.5772/62830
Miller, F. A., Fundo, J. F., Garcia, E., Santos, J. R., Silva, C. L. M., & Brandão, T. R. S. (2020). Physicochemical and bioactive caracterisation of edible and waste parts of “Piel de Sapo” melon. Horticulturae, 6(4), 60. https://doi.org/10.3390/horticulturae6040060
Moraes, D. P., Machado, M. L., Farias, C. A. A., Barin, J. S., Zabot, G. L., Lozano-Sánchez, J., Ferreira, D. F., Vizzotto, M., Leyva-Jimenez, F. J., Da Silveira, T. L., Ries, E. F., & Barcia, M. T. (2020). Effect of microwave hydrodiffusion and gravity on the extraction of phenolic compounds and antioxidant properties of blackberries (Rubus spp.): Scale-up extraction. Food and Bioprocess Technology, 13(12), 2200–2216. https://doi.org/10.1007/s11947-020-02557-z
Moreno-González, M., & Ottens, M. (2021). A structured approach to recover valuable compounds from agri-food side streams. Food and Bioprocess Technology, 14(8), 1387–1406. https://doi.org/10.1007/s11947-021-02647-6
Muthukumaran, C., Banupriya, L., Harinee, S., Sivaranjani, S., Sharmila, G., Rajasekar, V., & Kumar, N. M. (2017). Pectin from muskmelon (Cucumis melo var. reticulatus) peels: Extraction optimization and physicochemical properties. Biotech 3, 7(1), 66. https://doi.org/10.1007/s13205-017-0655-3
Niknam, S. M., Kashaninejad, M., Escudero, I., Sanz, M. T., Beltrán, S., & Benito, J. M. (2021). Valorization of olive mill solid residue through ultrasound-assisted extraction and phenolics recovery by adsorption process. Journal of Cleaner Production, 316, 128340. https://doi.org/10.1016/j.jclepro.2021.128340
Nuutinen, E. -M., Willberg-Keyriläinen, P., Virtanen, T., Mija, A., Kuutti, L., Lantto, R., & Jääskeläinen, A. -S. (2019). Green process to regenerate keratin from feathers with an aqueous deep eutectic solvent. RSC Advances, 9(34), 19720–19728. https://doi.org/10.1039/C9RA03305J
Ozgen, S., & Sarıoglu, K. (2013). Synthesis and characterization of acrylonitrile-co-divinylbenzene (AN/DVB) polymeric resins for the isolation of aroma compounds and anthocyanins from strawberry. Food and Bioprocess Technology, 6(10), 2884–2894. https://doi.org/10.1007/s11947-012-0969-4
Ozturk, B., Parkinson, C., & Gonzalez-Miquel, M. (2018). Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Separation and Purification Technology, 206, 1–13. https://doi.org/10.1016/j.seppur.2018.05.052
Panić, M., Gunjević, V., Cravotto, G., & Radojčić Redovniković, I. (2019). Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chemistry, 300, 125185. https://doi.org/10.1016/j.foodchem.2019.125185
Pérez-Armada, L., Rivas, S., González, B., & Moure, A. (2019). Extraction of phenolic compounds from hazelnut shells by green processes. Journal of Food Engineering, 255, 1–8. https://doi.org/10.1016/j.jfoodeng.2019.03.008
Raji, Z., Khodaiyan, F., Rezaei, K., Kiani, H., & Hosseini, S. S. (2017). Extraction optimization and physicochemical properties of pectin from melon peel. International Journal of Biological Macromolecules, 98, 709–716. https://doi.org/10.1016/j.ijbiomac.2017.01.146
Ralet, M. -C., Cabrera, J. C., Bonnin, E., Quéméner, B., Hellìn, P., & Thibault, J. -F. (2005). Mapping sugar beet pectin acetylation pattern. Phytochemistry, 66(15), 1832–1843. https://doi.org/10.1016/j.phytochem.2005.06.003
Rico, X., Gullón, B., Alonso, J. L., & Yáñez, R. (2020a). Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Research International, 132, 109086. https://doi.org/10.1016/j.foodres.2020.109086
Rico, X., Gullón, B., & Yáñez, R. (2020b). Environmentally friendly hydrothermal processing of melon by-products for the recovery of bioactive pectic-oligosaccharides. Foods, 9(11), 1702. https://doi.org/10.3390/foods9111702
Rico, X., Nuutinen, E. -M., Gullón, B., Pihlajaniemi, V., & Yáñez, R. (2021). Application of an eco-friendly sodium acetate/urea deep eutectic solvent in the valorization of melon by-products. Food and Bioproducts Processing, 130, 216–228. https://doi.org/10.1016/j.fbp.2021.10.006
Rodríguez-Juan, E., Rodríguez-Romero, C., Fernández-Bolaños, J., Florido, M. C., & Garcia-Borrego, A. (2021). Phenolic compounds from virgin olive oil obtained by natural deep eutectic solvent (NADES): Effect of the extraction and recovery conditions. Journal of Food Science and Technology, 58(2), 552–561. https://doi.org/10.1007/s13197-020-04567-3
Rolim, P. M., Seabra, L. M. J., & de Macedo, G. R. (2019). Melon by-products: Biopotential in human health and food processing. Food Reviews International, 36(1), 15–38. https://doi.org/10.1080/87559129.2019.1613662
Saha, S. K., Dey, S., & Chakraborty, R. (2019). Effect of choline chloride-oxalic acid based deep eutectic solvent on the ultrasonic assisted extraction of polyphenols from Aegle marmelos. Journal of Molecular Liquids, 287, 110956. https://doi.org/10.1016/j.molliq.2019.110956
Silva, E. M., Pompeu, D. R., Larondelle, Y., & Rogez, H. (2007). Optimisation of the adsorption of polyphenols from Inga edulis leaves on macroporous resins using an experimental design methodology. Separation and Purification Technology, 53(3), 274–280. https://doi.org/10.1016/j.seppur.2006.07.012
Silva, M. A., Albuquerque, T. G., Alves, R. C., Oliveira, M. B. P. P., & Costa, H. S. (2020). Melon ( Cucumis melo L.) by-products: Potential food ingredients for novel functional foods? Trends in Food Science & Technology, 98, 181–189. https://doi.org/10.1016/j.tifs.2018.07.005
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16(3), 144–158.
Soto, M. L., Conde, E., González-López, N., Conde, M. J., Moure, A., Sineiro, J., Falqué, E., Domínguez, H., Núñez, M. J., & Parajó, J. C. (2012). Recovery and concentration of antioxidants from winery wastes. Molecules, 17(3), 3008–3024. https://doi.org/10.3390/molecules17033008
Thibault, J. -F., & Ralet, M. -C. (2003). Physico-chemical properties of pectins in the cell walls and after extraction. In F. Voragen, H. Schols, & R. Visser (Eds.), Advances in Pectin and Pectinase Research (pp. 91–105). Springer, Netherlands. https://doi.org/10.1007/978-94-017-0331-4_7
Tsuru, C., Umada, A., Noma, S., Demura, M., & Hayashi, N. (2021). Extraction of pectin from Satsuma mandarin orange peels by combining pressurized carbon dioxide and deionized water: A green chemistry method. Food and Bioprocess Technology, 14(7), 1341–1348. https://doi.org/10.1007/s11947-021-02644-9
Vella, F. M., Cautela, D., & Laratta, B. (2019). Characterization of polyphenolic compounds in cantaloupe melon by-products. Foods, 8(196), 1–10. https://doi.org/10.3390/foods8060196
Wang, Z., Li, S., Ge, S., & Lin, S. (2020). Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. Journal of Agricultural and Food Chemistry, 68(11), 3330–3343. https://doi.org/10.1021/acs.jafc.9b06574
Wu, D., Zheng, J., Mao, G., Hu, W., Ye, X., Linhardt, R. J., & Chen, S. (2020). Rethinking the impact of RG-I mainly from fruits and vegetables on dietary health. Critical Reviews in Food Science and Nutrition, 60(17), 2938–2960. https://doi.org/10.1080/10408398.2019.1672037
Yang, Q., Zhao, M., & Lin, L. (2016). Adsorption and desorption characteristics of adlay bran free phenolics on macroporous resins. Food Chemistry, 194, 900–907. https://doi.org/10.1016/j.foodchem.2015.08.070
Zhang, F., Zhang, L., Chen, J., Du, X., Lu, Z., Wang, X., Yi, Y., Shan, Y., Liu, B., Zhou, Y., Wang, X., & Lü, X. (2022). Systematic evaluation of a series of pectic polysaccharides extracted from apple pomace by regulation of subcritical water conditions. Food Chemistry, 368(22), 130833. https://doi.org/10.1016/j.foodchem.2021.130833
Zhu, R., Wang, C., Zhang, L., Wang, Y., Chen, G., Fan, J., Jia, Y., Yan, F., & Ning, C. (2019). Pectin oligosaccharides from fruit of Actinidia arguta: Structure-activity relationship of prebiotic and antiglycation potentials. Carbohydrate Polymers, 217, 90–97. https://doi.org/10.1016/j.carbpol.2019.04.032
Acknowledgements
The authors would like to thank the Centro de Apoio Científico-Tecnolóxico á Investigación (C.A.C.T.I.) for performing the nitrogen and metals determination, and the HPLC–PDA–MS/MS, FTIR and HPAEC-PAD assays.
Funding
Open Access funding provided thanks to the CRUE-CSIC (Universidade de Vigo/CISUG) agreement with Springer Nature. This study is financially supported by “Xunta de Galicia” (GRC ED431C 2018/47, Centro Singular de Investigación Biomédica “CINBIO” and Project ED431F 2020/03). These projects are partially funded by the FEDER Program of the European Union (“Unha maneira de facer Europa”). Dr. Beatriz Gullón is supported by the Spanish Ministry of Economy and Competitiveness through postdoctoral grant (Reference RYC2018-026177-I). Predoctoral grant (reference ED481A-2018/300) with financial support from the “Xunta de Galicia” is provided to Xiana Rico.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rico, X., Gullón, B. & Yáñez, R. A Comparative Assessment on the Recovery of Pectin and Phenolic Fractions from Aqueous and DES Extracts Obtained from Melon Peels. Food Bioprocess Technol 15, 1406–1421 (2022). https://doi.org/10.1007/s11947-022-02823-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02823-2