Abstract
Green coffee processing has been hindered by low oil extraction yields from mechanical pressing and the need of using flammable and hazardous solvents for defatting the protein-rich cake before subsequent protein extraction. To replace the use of flammable solvents and enable the simultaneous extraction of lipids and proteins from green coffee beans at reduced water usage, a multistage countercurrent extraction process was scaled up from 0.05 to 1.14 kg and evaluated regarding protein and oil extractability, physicochemical and functional properties of the extracted protein, and oil recovery. Enzymatic extraction increased protein extractability by ~13% while achieving similar oil extractability when not using enzymes (55%). Proteolysis resulted in the release of smaller proteins with reduced surface hydrophobicity and higher solubility at acidic pH (3.0–5.0). The physicochemical changes observed due to proteolysis resulted in the formation of emulsions with reduced resistance against enzymatic and chemical demulsification strategies, enhancing the recovery of the extracted oil (48.6–51.0%). Proteolysis did not alter the high in vitro digestibility of green coffee proteins (up to 99%) or their emulsifying properties at most pH values evaluated. However, proteolysis did reduce the foaming properties of the hydrolysates compared with larger molecular weight proteins. These findings revealed the impact of extraction conditions on the extractability and structural modifications altering the functionality of green coffee proteins and the synergistic impact of extraction and demulsification strategies on the recovery of the extracted oil, paving the way for the development of structure–function processes to effectively produce green coffee proteins with desired functionality.
Similar content being viewed by others
Introduction
Coffee beans have been primarily used to produce beverages worldwide. However, because of the potential health benefits associated with green coffee oil such as antioxidant activity, protection against damage caused by UVB radiation, regeneration of lipids from the corneum stratum, besides the good emollient properties (Voytena et al., 2017), coffee beans have also been redirected to the oil industry. Furthermore, growing interest in the production and utilization of green coffee protein extracts has been recently fueled by the whole plant-based protein concept, which brings additional benefits associated with the presence of soluble phytoantioxidants (Applied Food Sciences, 2019; Oliveira et al., 2019a; Prandi et al., 2021; Siegner, 2019). Green coffee oil is frequently extracted by cold mechanical pressing, a process known for having low extraction efficiency for low oil content matrices such as coffee beans. Therefore, it generates a cake byproduct containing proteins and unextracted lipids, the latter requiring subsequent removal by the use of neurotoxic/flammable solvents. This not only raises environmental/safety concerns but can negatively affect the quality of the extracted oil (Chemat et al., 2019). Therefore, current extraction practices can hinder the potential use of this fraction for cosmetic and food applications (Oliveira et al., 2019b).
The use of proteins as ingredients in food systems is of key importance as they possess numerous functional properties (e.g., gelation, solubility, emulsification, and foaming capacity) (Zayas, 1997) of interest for the production of nutritious and functional foods. Among the wide range of plant-based proteins currently being evaluated for industrial applications, green coffee protein is one of the least evaluated by current research, evidencing a knowledge gap about the impact of extraction and fractionation strategies on their physicochemical and functional properties, a required step to identify potential applications for green coffee protein.
The use of aqueous (AEP) and enzymatic extraction processes (EAEP) is an effective green extraction approach that can address the low extraction yields from mechanical pressing and the subsequent use of flammable solvents to further defat the residual cake. Importantly, it enables the simultaneous extraction of water-soluble (i.e., proteins, polyphenols, and carbohydrates) and insoluble (i.e., lipids) compounds from different matrices such as soybeans (De Moura et al., 2009), peanuts (Jiang et al., 2010), sunflower (de Aquino et al., 2019), and yellow mustard (Tabtabaei & Diosady, 2013). In addition to achieving high extraction yields, these techniques present operational flexibility, and low capital investment, energy consumption, and operational costs (Wu et al., 2009). Importantly, AEP and EAEP of oilseeds and some tree nuts have been shown to produce oils of similar quality to mechanically expressed oils (Mechqoq et al., 2021; Polmann et al., 2019; Soto et al., 2007). However, the high water usage required to achieve high extractability is an unquestionable challenge of aqueous extraction processes, often leading to the generation of a high quantity of aqueous extracts that need to be handled, centrifuged, and spray-dried to produce protein powder for subsequent applications (Souza Almeida et al., 2021; Souza et al., 2019).
Our research group recently demonstrated high oil (48%) and protein (70%) extraction yields from green coffee when using enzymes in the extraction. However, the high extractability achieved was only possible due to the use of a large amount of water (1:17.5 solids-to-liquid ratio, SLR), a common challenge when performing single-stage extractions. To circumvent this issue, we developed a two-stage countercurrent enzymatic extraction process that successfully reduced the water usage in the process by 60% while increasing oil and protein extractability to 58 and 72%, respectively (Souza Almeida et al., 2021). However, because the feasibility of the process developed relies not only on achieving high extraction yields but also on maximizing the recovery of the extracted oil and production of proteins with desired technological functions, we decided to evaluate strategies to destabilize the emulsion produced by the process to free the extracted oil and to characterize the functional properties of the extracted green coffee protein to identify potential industrial applications.
Since previous research has been performed only at the lab scale (50 g of green coffee flour) and oil recovery and protein functionality have not been evaluated yet, the two-stage countercurrent AEP and EAEP processes were scaled up to find potential issues that might occur at a larger-scale and to produce enough cream (emulsion) and protein extracts (skim) for oil recovery and functional analyses. Therefore, the specific objectives of the present research were to (i) determine the impact of scaling up the two-stage countercurrent extraction (with and without enzyme) from 0.05 to 1.14 kg of green coffee flour on oil and protein extractability, (ii) evaluate the impact of enzymatic extraction on the recovery of the extracted coffee oil (entrapped in an oil in water emulsion) by enzymatic and chemical demulsification strategies, and to (iii) assess the influence of enzymatic extraction on the physicochemical and functional properties of the extracted protein. These data will help elucidate the impact of proteolysis in the countercurrent extraction process on the technological properties of green coffee proteins and will determine for the first time the integrated impact of extraction and recovery methods on final oil recovery from green coffee beans.
Material and Methods
Green Coffee and Enzyme
Arabica green coffee beans produced in the Brazilian Cerrado (Genuine Origin, NY, USA) were used in the extractions. The beans were ground using a blender (Vitamix VM0103, Cleveland, OH, USA) at the maximum speed to obtain the flour (9.40 ± 0.35% oil, 12.74 ± 0.09% protein, and 5.29 ± 0.22% moisture content) for subsequent use in the extractions. The commercial alkaline endoprotease from Bacillus licheniformis (Danisco, Rochester, NY, USA), presenting enzyme activity 580,000–650,000 DU/g in the pH range 7.5–10.5, and temperature range 40–70 °C, was used to assist the extractions.
Scaling Up the Two-Stage Countercurrent Extraction Process
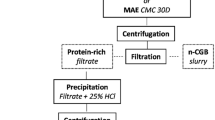
Except for the increase in the amount of green coffee flour from 0.05 to 1.14 kg, the two-stage countercurrent extraction process was performed as previously described by Souza Almeida et al. (2021). Initially, the coffee flour was first extracted by dispersing 1.14 kg of the green coffee flour in distilled water to reach a 1:7 (w/v) solids-liquid ratio (SLR), without enzyme addition. The extraction was performed at 50 °C in a 10 L jacketed glass reactor (CG-1965-610 M–Chemglass Life Sciences LLC, Vineland, NJ, USA) using mechanical agitation at 120 rpm for 30 min, and at pH 9.0 (adjusted with 1 M NaOH). After extraction, the slurry followed centrifugation at 4000 × g for 30 min at 4 °C to remove the insoluble fraction (Insoluble A) (Fig. 1), which was subjected to a second extraction. The insoluble A was dispersed in water to achieve 1:7 SLR and 0.1% (w/w) of alkaline protease was added to assist the extraction, which was carried out under the same conditions as the first extraction. Following the centrifugation of the second extraction slurry, the liquid fraction (Skim B + Cream B) obtained was used to slurry the first extraction on the subsequent run with fresh incoming flour (Fig. 1). Each two-stage extraction was considered as one run and was sequentially replicated three times. Only liquid fractions (Cream A + Skim A) generated from the third run (when enzymes had been adequately recycled between the runs) were separated overnight at 4 °C into cream and skim fractions for further proximate characterization, demulsification, and functional analyses. The skim fraction (protein-rich phase) is further referred to as the extract. Oil (OE) and protein (PE) extraction yields were determined according to equations (Eqs. (1) and (2)) and the distribution among the fractions was obtained by Eq. (3) to (4). To gain a deeper understanding of the impact of enzyme use during the extraction on the functional and physicochemical properties of the extracted compounds, a two-stage countercurrent extraction without enzyme use (AEP) was also performed as above described.
Understanding the Integrated Impact of Extraction and Demulsification Conditions on Oil Recovery
To recover the extracted oil entrapped in the oil-rich emulsion (cream A), chemical and enzymatic demulsification strategies were evaluated to destabilize the emulsions generated by the countercurrent EAEP and AEP (without enzyme use) (Fig. 1). Oil recovery was attempted by incubating 20 g of well-mixed cream samples in a 50 °C water bath under constant stirring at 600 rpm (de Souza et al., 2020a). Chemical demulsification consisted of adjusting the cream pH to 3.5 (zero net charge for coffee proteins) (Wen et al., 2021), while the enzymatic demulsification was carried out by adjusting the cream pH to 9.0 (optimal condition for alkaline protease activity) with 1 N NaOH, followed by the addition of varying amounts of alkaline protease (0.5, 1.0 and 2.0% w/w). A control sample was also prepared without enzyme and at the original pH of the creams. All samples were incubated for 90 min, followed by cooling in an ice bath for 15 min, and subsequent centrifugation at 3000 × g for 15 min at 25 °C. Afterward, the free oil layer was removed, and, for quantitative purposes, hexane was used to wash out the remaining oil. The hexane-lipid fraction was submitted to air drying and the oil recovery yield was obtained (Eq. (5)) (de Moura et al., 2011):
Physicochemical Properties of Protein Extracts from the Two-Stage Countercurrent Extraction
Proximate analyses
To calculate oil and protein extractability, cream demulsification yields, and to further characterize the samples for subsequent analyses, all fractions derived from the extraction process (cream, insoluble, skim, and coffee flour) were analyzed for dry matter, oil (performed by acid hydrolysis), and protein contents (AOAC, 1990). The latter was measured by the Dumas method using a Nitrogen Analyzer (Vario MAX cube, Elementar Analysensysteme GmbH, Langenselbold, Germany) and a protein conversion factor of 5.24 (Souza Almeida et al., 2021). Ash determination of skim fractions was performed following the AOAC protocol (AOAC, 1990). All analyses were performed in triplicate for each processing replicate sample (n = 3).
Surface Charge–Zeta Potential
The surface charge of the freeze-dried protein extracts was determined by using a Zetasizer Nano-ZS (Malvern, UK). Protein extract solutions (0.01% w/w) were prepared with Milli-Q water at different pH values (2.0, 3.0, 3.5, 4.0, 5.0, 7.0, and 9.0), by adding either 0.01 N HCl or 0.01 N NaOH solutions. Zeta potential measurements were carried out at least in triplicate for each processing replicate (n = 3).
Protein Surface Hydrophobicity (H0)
Protein extracts were characterized according to protein surface hydrophobicity by using the 1-anilino-8-naphthalenesulfonate (ANS) fluorescence probe, following the protocol described by Zhang et al. (2013b), with modifications. For this, AEP/EAEP extracts were initially diluted with 0.01 M sodium phosphate buffer solution (pH 7.0) to obtain variable protein concentrations varying from 0.022 to 0.22 mg/mL. Next, 250 µL of the diluted extract was placed into a 96-well plate, followed by the addition of 1.25 µL of ANS solution (8.0 mM in 0.01 M phosphate buffer, pH 7.0, solution). The fluorescence intensity was measured in a microplate reader (SpectraMax iD5 Multi-Mode Microplate Readers, Molecular Devices, San Jose, California, USA) at an excitation wavelength of 390 nm and emission wavelength of 470 nm (both with a slit width of 5 nm). Protein hydrophobicity was determined by linear regression analysis, considering the slope (So) of fluorescence intensity vs. protein concentration. H0 measurements were performed six times for each processing replicate sample (n = 3).
Degree of Hydrolysis (DH) of AEP and EAEP Protein Extracts
The impact of the two-stage countercurrent extraction process on the degree of protein hydrolysis, with and without enzyme use, was determined (Nielsen et al., 2001). The standard solution was composed of a 0.9516 meqv/L l-serine solution and the control contained distilled water. Color interference from the extracts was taken into account by using a sample background containing distilled water instead of the o-phthaldialdehyde (OPA) reagent. Protein quantification (%) was determined by using a Nitrogen Analyzer (Vario MAX cube, Elementar Analysensysteme GmbH, Langenselbold, Germany) and the DH was obtained based on the following equations (Eqs. (6) and (7)):
The constants α, β, and htotal values correspond to 1.0, 0.4 (Nielsen et al., 2001), and 7.84 (calculated through the amino acid composition of the coffee beans), respectively.
Protein Molecular Weight Distribution by Electrophoresis
The molecular weight distribution of the proteins extracted by the two-stage countercurrent AEP and EAEP was characterized by electrophoresis using a sodium dodecyl sulfate–polyacrylamide gel. Briefly, aliquots of liquid AEP and EAEP extracts were mixed with (1:1 v/v) Laemmli solution containing β-mercaptoethanol (Bio-Rad, Hercules, CA, USA). Then, samples were vortexed and heated in a water bath at 95 °C for 5 min, for protein denaturation (Laemmli, 1970). After that, 30 µg of protein aliquots were placed in each well onto a precast 12% acrylamide gel (CriterionTM TGX Precast Gels, Bio-Rad, Hercules, CA, USA) to perform the separation at 200 V, room temperature, for 1 h. The running buffer consisted of a Tris–HCl buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3) (Bio-Rad, Hercules, CA, USA) and the Precision Plus Protein Dual Color (10–250 kDa) (Bio-Rad, Hercules, CA, USA) was used as standard. The relative abundance and distribution of proteins were analyzed using a Gel DOCTM EZ Imager system and Image Lab software (Bio-Rad, Hercules, CA, USA).
Nutritional and Functional Properties of the Protein-Rich Extracts
In Vitro Protein Digestibility
AEP and EAEP protein extracts were tested against simulated digestion fluids as described previously (Bornhorst & Singh, 2013; de Souza et al., 2020b). Briefly, 5 mL of the extract was mixed with 3.33 mL of simulated saliva fluid (SSF) (final concentration: 75 U/mL alpha-amylase, 1 mg/mL mucin, 0.117 mg/mL NaCl, 0.149 mg/mL KCl, and 2.1 mg/mL NaHCO3) at pH 7.0 and vortexed. Then, 6.67 mL of simulated gastric fluid (SGF) (Final concentration: 2000 U/mL pepsin, 1.5 mg/mL gastric mucin, and 8.8 mg/mL NaCl) was added and the pH was adjusted to 3.0. Samples were incubated in a water bath under stirring (140 rpm) for 2 h at 37 °C. Next, the simulated intestinal fluid (SIF) (final concentration: 800 U/mL pancreatin, 10 mg/mL bile extract, and 16.8 mg/mL NaHCO3) was added (1:1 v/v), following pH adjustment to 7.0, and incubation under the same previous conditions. Sample aliquots (500 µL) were collected at the end of each digestion phase to evaluate the molecular weight profile (“Protein Molecular Weight Distribution by Electrophoresis” section) of the proteins contained in the skim fractions and, to stop the reaction, samples were heated at 85 °C for 3 min. Proteins were precipitated by the addition of a 24% (w/v) trichloroacetic acid solution at the 1:1 (v/v) proportion and they followed centrifugation at 4000 rpm for 30 min at 4 °C to obtain the precipitate (pellet). The latter was used for nitrogen quantification before and after the digestion using a Nitrogen Analyzer (Vario MAX cube, Elementar Analysensysteme GmbH, Langenselbold, Germany). The protein digestibility was obtained as follows (Eq. (8)):
where NPbefore corresponds to the protein content before digestion, NPafter is the protein content after digestion, and NPblank is the enzyme blank.
Solubility of Green Coffee Protein-Rich Extracts
The solubility of lyophilized AEP and EAEP protein extracts (Labconco, Kansas, Missouri, USA) was determined according to Souza et al. (2019). For this, 10 mL aliquots containing 2% (w/v) of protein extract solutions were placed in a 30 mL beaker for the analysis. The sample pH was then adjusted to different values (2.0, 3.0, 3.5, 4.0, 5.0, 7.0, and 9.0) using either 1 N HCl or 1 N NaOH solutions. After stirring at 150 rpm for 1 h at 20 °C, the dispersions were next centrifuged for 10 min at 10,000 × g and 20 °C. A Nitrogen Analyzer (Vario MAX cube, Elementar Analysensysteme GmbH, Langenselbold, Germany) and a protein conversion factor of 5.24 (Souza Almeida et al., 2021) were used to determine the protein content of the freeze-dried powders and supernatants. Each sample was analyzed in triplicate. Protein solubility (%) was obtained as follows (Eq. (9)):
Color Determination
To quantify the changes in the color of the extracts during the solubility assay as a function of pH, the parameters L* (lightness), a*, and b* were obtained, and the hue angle (H) was calculated (Eq. (10)). For the measurements, a ColorFlex spectrophotometer (Hunter Lab ColorFlex 45/0, CX2478, Reston, VA, USA) was used, with D65 as the illuminant and 10° as the observer angle. The measurements were recorded at least in triplicate.
Emulsifying Properties of Green Coffee Protein-Rich Extracts
Emulsifying properties of AEP and EAEP protein extracts (skims) were determined according to Jamdar et al. (2010) and Pearce and Kinsella (1978), with modifications. For this, 2% (w/v) skim aqueous solutions were prepared and 15 mL aliquots were transferred to 100 mL beakers. The solution pH was adjusted to pH 2.0, 3.0, 3.5, 4.0, 5.0, 7.0, and 9.0 by using either 1 N HCl or 1 N NaOH. Then, 5 mL of soybean oil was added to the skim solutions and the mixtures were homogenized using a Polytron PT 2500 homogenizer (Kinematica AG, Lucerne, Switzerland) at 20,000 rpm for 1 min. Fifty microliters aliquots were collected from the bottom of the container and mixed with 10 mL of 0.1% sodium dodecyl sulfate (SDS) solution at 0 and 10 min after homogenization. The absorbances of the diluted solutions were recorded at 500 nm using a spectrophotometer (G10 S UV–Vis; Thermo Fisher Scientific, Madison, WI, USA), and the emulsifying activity index (EAI) and emulsion stability (ES) were obtained (Eqs. (11) and (12)):
where Abs0 is the absorbance of the diluted emulsion measured immediately after homogenization, DF is the dilution factor (200), φ is the oil fraction (0.25), C is the weight of protein per volume (g/mL), ∆t is the time interval (10 min), and Abs10 is the absorbance after 10 min. Samples were analyzed at least in triplicate.
Foaming Properties of Green Coffee Protein-Rich Extracts
Foaming capacity (FC) and stability (FS) of AEP and EAEP protein extracts were determined according to Jamdar et al. (2010). Twenty microliters of previously prepared 2% (w/v) freeze-dried skims aqueous solutions were transferred to 100 mL beakers and the pH of the solutions was adjusted to 2.0, 3.0, 3.5, 4.0, 5.0, 7.0, and 9.0 by using either 1 N HCl or 1 N NaOH. Skim samples were homogenized to incorporate air using a Polytron PT 2500 homogenizer (Kinematica AG, Lucerne, Switzerland) at 16,000 rpm, for 2 min at ambient temperature. The whipped sample was immediately transferred into a 100 mL cylinder and the total foam volume was read after 30 s to determine foaming capacity (FC) (Eq. (13)) and after 10, 30, and 60 min for foaming stability (FS) (Eq. (14)). Each sample was evaluated in triplicate.
where VA is the volume after whipping the skim solutions (mL), VB is the volume before the solutions were whipped (mL), and Vt is the volume after 10, 30, and 60 min.
Statistical Analyses
Each processing replicate (n = 3) was analyzed at least in triplicate for all analyses. Analysis of variance (ANOVA) followed by Tukey’s tests or Student’s t test was performed using the TIBCO Statistica software (TIBCO Software Inc, Palo Alto, CA, USA) at the 5% level of significance (p < 0.05).
Results and Discussion
Effects of Scaling Up the Two-Stage Countercurrent AEP and EAEP on Coffee Protein and Oil Extractability
Considering the potential effect of processing scale-up on extraction yields and the need of producing larger volumes of protein-rich extracts and emulsion for functional, biological, and oil recovery studies, the two-stage countercurrent AEP and EAEP from green coffee was scaled up by 22 times, with respect to mass. Table 1 shows protein and oil extractability and their distribution among fractions. AEP and EAEP resulted in similar total oil extraction yields (average ~55%) and oil distribution among the phases, except for the EAEP skim, which had a higher oil yield (12.2 vs. 8.48%). Higher oil in the EAEP extract might be related to the release of smaller peptides by proteolysis, which could enhance emulsion formation and reduce the migration of lipids from the extract into the cream fraction. The reduced molecular weight and higher solubility of the hydrolysates facilitate their diffusion and interaction at the oil–water interface, often improving their emulsifying properties (Wu et al., 1998). Although small peptides can lead to the formation of emulsions with reduced stability, they are often more efficient to lower the surface tension compared with larger or native peptides (Ricardo et al., 2021).
Therefore, although the use of enzyme in the extraction brings no additional improvement in oil extractability, it does produce an extract with higher oil content (12.2 vs. 8.48%) which could, in turn, affect the solubility of the extracted protein and reduce the recovery of the extracted oil as there is no method yet available to recover the diluted oil from the extract (Dias et al., 2020). Similar oil extraction yields and distribution were observed at a smaller scale (0.05 kg of flour) (Souza Almeida et al., 2021), indicating that coffee oil extractability and distribution were not affected by processing scale-up.
Enzymatic extraction significantly improved protein extractability from 64.5 to 72.8% (Table 1). Higher protein extractability in the enzyme-assisted process can be explained by the ability of proteases to release smaller and often more soluble protein fragments or peptides into the extraction medium (Pojić et al., 2018). Nearly all the extracted protein (95% for AEP and 85% for EAEP) was present in the extracts (61.2% AEP extract vs. 69.8% EAEP extract yield), with only 5% (AEP) and 3.5% (EAEP) of the extracted protein being present in the cream fractions.
Higher protein content in the protein-rich extract is desirable as it boosts the amount of protein that can be recovered for subsequent industrial applications. In addition, because protein can diffuse to the oil–water interface, adsorb, and rearrange themselves to form an elastic protein film around oil droplets (Damodaran et al., 2008), its presence in the cream fraction can impact the emulsion stability, thus altering the overall recovery of the extracted oil. Even though different agitation systems (impeller vs. stir plate) were employed at small and larger-scale, similar protein extractability was achieved (~72%) (Souza Almeida et al., 2021).
Cream Demulsification
While aqueous and enzyme-assisted extractions processes are effective in simultaneously extracting lipids and proteins from several matrices without the use of flammable solvents, nearly all the extracted oil is entrapped in the emulsion, which needs to be further destabilized to free the oil for commercial applications (Dias et al., 2020). Because oil recovery can be affected by both extraction and cream demulsification conditions, we evaluated the integrated impact of the two-stage countercurrent extraction process (AEP and EAEP) and enzymatic and chemical demulsification approaches on the recovery of green coffee oil (Table 2).
The recovery of the AEP cream oil was significantly (p < 0.05) enhanced from 33% (control) to 43.6–52.2% by the use of enzyme concentrations ranging from 0.5 to 2.0% during the demulsification process. However, no improvement in oil recovery was observed by the chemical demulsification approach (33%), indicating that the pH shift to the isoelectric protein of green coffee proteins was not an effective strategy to destabilize the AEP emulsion. Nonetheless, both demulsification approaches were similarly effective to increase oil recovery from the EAEP cream from 35% (control) to 51% (chemical approach), and 43.6–52.8% (enzymatic approach), which demonstrates that a simple chemical demulsification process can achieve high recovery yields because of the upstream impact of the protease used during the extraction. Moreover, the EAEP process did not affect the cream composition regarding oil and protein content (Table 2).
Overall, the AEP cream (obtained without enzyme use during the extraction) was more stable toward chemical demulsification than the EAEP cream (containing more extensively hydrolyzed proteins), but it was as susceptible to enzymatic demulsification as the EAEP cream. Considering that the chemical demulsification mechanism relies on adjusting the pH of the emulsion to the isoelectric point of proteins, thus causing protein agglomeration and consequently oil release (Damodaran et al., 2008), the results presented herein highlight the stabilizing role of more native proteins in the AEP cream. However, enzymatic demulsification, which relies on the ability of proteases to reduce the molecular size of the interfacial proteins and rigidity of the oil droplet at the interface (Chabrand & Glatz, 2009; Mat Yusoff et al., 2015), was effective to enhance coalescence and free oil recovery from the AEP cream. Therefore, stable emulsions seem to be better destabilized by the enzymatic breaking down of proteins into smaller protein fragments and peptides. These partially hydrolyzed proteins, usually presenting lower surface hydrophobicity (Dias et al., 2020), can alter the interfacial film integrity and thus, enhance oil coalescence (Zhang et al., 2013b). Our results agree with the ones described for almond cake (de Souza et al., 2020a), peanut (Zhang et al., 2013a), and soybeans (Jung et al., 2009), where emulsions containing AEP proteins presented higher stability. The results presented herein demonstrate that the two-stage countercurrent EAEP followed by chemical or enzymatic demulsification of the cream is an effective strategy to maximize both the extraction of lipids and proteins and to enhance the recovery of the extracted oil from the emulsion.
Protein-Rich Extract Characterization
Proximate Composition of the Protein Extracts
Considering that the extract composition can affect its functional properties, the chemical composition of the extracts was determined (Table 3). Protein and carbohydrates were the main components of green coffee extracts, followed by ashes, and lipids. The high content of carbohydrates found in the extracts (56 to 60%) agrees with the coffee composition, where 50% of the weight of green coffee beans is composed of polysaccharides (mainly cellulose, galactomannans, and arabinogalactan-proteins) (Redgwell & Fischer, 2006). The higher protein extractability of the EAEP, reflected by the higher protein content in the EAEP extract (24.5 vs. 22.8% w/w), likely generated a more porous structure due to the diffusion of the proteins into the extraction medium, enhancing the washing of the oil into the extraction medium, as reflected by the higher oil content of the EAEP extract (3.84 vs. 2.41% w/w). Moreover, smaller peptides generated during EAEP probably trapped small oil droplets in the skim more efficiently, hindering the migration of extract oil to the cream fraction (Table 1).
Protein Surface Charge
The surface charges (ζ-potential) of AEP and EAEP protein extracts were mostly negative (−37 mV to −0.48 mV) at most pH values evaluated (pH 2.0–9.0), indicating that the isoelectric point (pI) of these proteins lay on the lower range of the pH values evaluated. However, proteolysis during the EAEP led to a shift in the pI of AEP protein from ~3.2 to 2.0 (Fig. 2). It is important to highlight that the extracts also contain carbohydrates and nitrogen-rich compounds such as chlorophyll and caffeine, which could influence the zeta potential values of the protein extracts (Thamnarathip et al., 2016).
In general, EAEP proteins exhibited higher absolute ζ-potential values than AEP proteins at the same pH value, evidencing that proteolysis can expose more ionizable amino and carboxyl groups, increasing the net charge of the protein, as previously demonstrated for almond proteins (de Souza et al., 2020a). Because attractive hydrophobic and repulsive electrostatic interactions play a fundamental role to establish protein–protein and protein-solvent interactions (Damodaran et al., 2008), the increase in the net charge of the proteins promoted by proteolysis might favor protein interactions with water, enhancing protein solubility (Zayas, 1997).
Protein Surface Hydrophobicity
The surface hydrophobicity (H0) of a protein is a good indicator of the protein’s ability to interact with other molecules. Hydrophobic interactions play an important role in protein stability, conformation, and functionality (Jiang et al., 2015). The effects of using enzyme in the two-stage countercurrent extraction process on the surface hydrophobicity of green coffee proteins are shown in Table 4. Enzymatic extraction decreased H0 from 625 to 530 (Table 4). Although partial hydrolysis can increase the H0 of a protein due to the exposure of hydrophobic groups previously buried inside the native protein molecule (Wu et al., 1998), as previously shown for the single-stage extraction of green coffee proteins, where enzymatic extraction increased H0 from 693.3 to 894.6 (Souza Almeida et al., 2021), more extensive proteolysis, as the one observed in the two-stage countercurrent EAEP (29.3%) (Table 4), can indeed decrease protein surface hydrophobicity. Reduced H0 is probably related to the disruption of hydrophobic bonds present at the protein surface and reduction of hydrophobic amino acid aggregates (Dias & de Moura Bell, 2022; Wu et al., 1998), which can negatively impact the interfacial tension and emulsion properties of the protein (Lam & Nickerson, 2013).
Protein Molecular Weight Distribution and Degree of Hydrolysis
The electrophoretic profile (Fig. 3B) and degree of hydrolysis (DH) (Table 4) of the two-stage countercurrent AEP and EAEP protein extracts were evaluated. The higher DH of the EAEP (29.3%) extract compared with the AEP extract (23.8%) reflects the use of enzyme in the two-stage countercurrent EAEP (Table 4). Although enzyme was not used during the aqueous extraction process, the high DH of the AEP proteins highlights the role of alkaline extraction conditions on protein hydrolysis. Prior work with soybeans reported a DH of 6.4% and 10.1% for the first and second extraction stages, respectively, when using two-stage countercurrent EAEP (De Moura et al., 2011). As expected, the results obtained for the two-stage process differ from our previous study using a single-stage extraction, where EAEP green coffee proteins exhibited a DH of 13.8% when the enzyme alkaline protease was employed during the extraction (Souza Almeida et al., 2021). The results presented herein emphasize the high susceptibility of green coffee proteins to hydrolysis in the presence of enzymes (EAEP) or absence of enzymes under alkaline conditions (AEP).
Effects of AEP and EAEP on A in vitro protein digestibility and B electrophoretic molecular weight distribution of proteins before and after digestion. Lane 1 indicates the molecular mass marker standard (10–250 kDa); lanes 2 and 6 represent AEP and EAEP samples before digestion; lanes 3, 4, and 5 and 7, 8, and 9 show samples after oral (SSF), gastric (SGF), and intestinal digestion (SIF) for AEP and EAEP samples, respectively; and lanes 10, 11, and 12 are the controls (SSF, SGF, and SIF). Different letters indicate statistical difference (p < 0.05) between the extractions. Red arrows indicate the enzyme band in the SGF and the blue arrows represent enzyme bands present in the SIF
SDS-PAGE shows the presence of protein fragments and peptides smaller than 10 kDa for AEP proteins (Fig. 3B). Although no enzyme was used during the extraction when producing the AEP extract, its high DH highlights the high solubility and breakdown of green coffee proteins under the extraction conditions employed, which involves two-stage countercurrent extractions at mild temperature (50 °C), high pH value (pH 9), and agitation (120 rpm), conditions that could intensify protein hydrolysis. The higher DH of the EAEP proteins (29.3%) correlates with the electrophoretic profile (Fig. 3B). EAEP proteins exhibited protein fragments close to 17 kDa and 12.5 kDa that were not present in the AEP extract. Moreover, protein fragments around 31 kDa and 20 kDa were present in both AEP and EAEP extracts.
Nutritional and Functional Properties of the Two-Stage Countercurrent AEP and EAEP Proteins
Protein In Vitro Digestibility
The effects of proteolysis on in vitro protein digestibility are shown in Fig. 3. High protein digestibility (> 97%) was found for both AEP and EAEP extracts, with no significant differences (p > 0.05) observed among the treatments. Despite the higher DH of the EAEP proteins, the addition of alkaline protease during the extraction did not increase the in vitro protein digestibility (Fig. 3A). Similar results were reported by Goertzen et al. (2021), where no improvements in protein digestibility were observed after proteolysis of chickpea protein isolates. Lower values of protein digestibility (86%) were reported by Jakubczyk et al. (2018) for green coffee flour. The higher values observed in our study can be attributed to the effects of the two-stage countercurrent extraction process on releasing smaller and more digestible proteins from coffee flour, which could facilitate the action of the digestive enzymes, instead of using the coffee flour. Our results were also higher than the ones reported for other plant protein extracts such as almonds, peas, and chickpeas (de Souza et al., 2020b; Jakubczyk et al., 2018; Laguna et al., 2017).
The SDS-PAGE profile showed that the salivary solution (SSF) did not affect the protein molecular distribution, indicating the lack of proteolysis in the oral phase. However, differences in the protein breakdown behavior along the gastric and intestinal digestion process were found. Coffee proteins were partially hydrolyzed by the pepsin enzyme in the gastric stage, resulting in the release of protein fragments/peptides smaller than 15 kDa for the AEP and 10 kDa for the EAEP (Fig. 3B). Pancreatin hydrolysis during the intestinal phase led to extensive digestion of coffee proteins, and only faint bands below 10 kDa can be observed (besides the band of the own pancreatin enzyme), in agreement with the high protein digestibility reported herein (~99%).
Solubility
Solubility is a highly relevant functional property since it influences other functionality of proteins (e.g., thickening, water retention, foaming, emulsifying, and gelling) and determines the ease of incorporation of the extracted protein into foods (Zayas, 1997). The role of the two-stage countercurrent AEP and EAEP on the solubility of green coffee protein as a function of pH (2 to 9) is shown in Fig. 4A. AEP and EAEP proteins presented the typical U-shaped Solubility vs. pH curve (Fig. 4A), with high and similar solubility values being observed for proteins obtained by both AEP and EAEP at very acidic or alkaline pH values (~73 to 90%, as observed for most proteins). However, reduced solubility for both AEP and EAEP protein extracts was observed within the pH range 3.0 to 4.0, suggesting reduced electrostatic repulsion between protein molecules, which could promote aggregation and precipitation via hydrophobic interactions, thus decreasing protein solubility (Damodaran et al., 2008). At the 3–4 pH range, proteolysis during the EAEP significantly increased protein average solubility from 44.1 to 55.4%, highlighting the impact of higher proteolysis (DH of 29.3 for EAEP and 23.8% for AEP) on the release of smaller protein fragments and peptides that are often more soluble. Similar results were reported by Jamdar et al. (2010) where the hydrolysis of peanut protein isolates (DH from 10 to 40%) significantly improved the protein solubility (from 40 to 90%) in the minimum solubility pH range of 4–6.
Solubility measurements agree with zeta potential values presented in Fig. 2, which show that AEP and EAEP proteins present a zero net charge at pH 3.2 and pH 2.0, respectively. The shift in the pI of the AEP proteins from 3.15 to 2.0 (hydrolyzed, EAEP) likely contributed to the higher solubility of EAEP proteins (55 vs. 44%) at pH 3–4. Increased solubility of EAEP proteins at pH 3–4 can be attributed to the release of smaller peptides (Table 4), ionizable groups (Souza et al., 2019), and the reduced surface hydrophobicity (H0) of the EAEP proteins (530.9 for EAEP vs. 625.3 for AEP) (Table 4). Therefore, by tailoring extraction conditions, coffee proteins with the desired functionality can be produced for applications in a variety of food-related applications.
The highest solubility values (up to 90%) were obtained from pH 7.0 to 9.0 for AEP/EAEP skim proteins (Fig. 4). At alkaline pH, stronger electrostatic repulsion between protein molecules (ZP ~ −30 mV, Fig. 2) overcomes their hydrophobic interactions (Damodaran et al., 2008; Zayas, 1997), which in turn increases their solubility into the aqueous medium. Thus, at alkaline pH, the additional benefits of proteolysis on protein solubility were not observed. Despite differences in the extraction processes used (single vs. two-stage extraction), the results presented herein are in agreement with the solubility of green coffee proteins produced by the single-stage extraction process at pH 4.0 (35 vs. 56% for AEP e EAEP, respectively) and 9.0 (> 88%) (Souza Almeida et al., 2021). The increased solubility of EAEP proteins at low pH values is of interest for food applications involving acidic mediums such as beverages, smoothies, and sports drinks.
Changes in the color parameters (L*, a*, b*, and H) of the protein extracts during the solubility assays at different pH values are described in Fig. 4B. Protein solutions went from red/orange colors (hue angle varying from 42 up to 83) in acidic media to a dark green appearance (hue angle varying from 119 up to 159) under neutral/alkaline pH. Color changes in the protein extracts could be related to the instability of compounds such as anthocyanins and chlorophylls released during the extractions, which are unstable toward pH changes, as previously reported for green coffee proteins (Souza Almeida et al., 2021). These results highlight the importance and need for adequate pH selection and development of strategies to mask the color of green proteins to enhance their applications in the food and nutraceutical industries.
Emulsifying Activity Index and Emulsification Stability Index
Emulsifying properties such as emulsification activity index (EAI) and emulsification stability index (ESI) are significantly affected by changes in the protein structure, size, composition, solubility, and surface hydrophobicity (Damodaran et al., 2008). Therefore, the impact of using enzymes in the two-stage countercurrent extraction process was evaluated on the EAI and ESI of the extracted protein (Fig. 5). Higher EAI and ESI values were observed at pH values outside the pH range 3.0 to 4.0 for both AEP and EAEP proteins, being similar at all pH values, except at pH 3.5, for which the AEP proteins presented a significantly higher EAI than the EAEP skim proteins (28.9 vs. 11.9 m2/g).
Although one could expect that the higher solubility of EAEP proteins at pH 3.5 would favor the migration and adsorption of proteins/peptides to the oil interface (De Almeida et al., 2014), the presence of small peptides can be associated with droplet coalescence, reducing the interfacial area and, consequently, the protein emulsifying properties (Damodaran et al., 2008). In addition, the reduced surface hydrophobicity of EAEP protein extracts (625.3 AEP and 530 EAEP, Table 4) might have contributed to its reduced EAI at pH 3.5. Surface hydrophobicity is the primary driving force for absorption (Lam & Nickerson, 2013), thus influencing protein absorption at the oil/water interface.
Highest EAI values (up to 76.6 m2/g) were found for both AEP and EAEP proteins at pH 5.0 and 9.0 with no significant differences (p > 0.05) among them. As observed for EAI, higher ESI was observed at pH 2.0 and alkaline pH. Overall, EAEP proteins presented higher ESI at alkaline conditions (30.3 min at pH 9.0), followed by pH 7.0 (Fig. 5). Emulsion stability is enhanced by electrostatic repulsion between emulsified droplets and hydration of the proteins adsorbed in the surrounding film (Zayas, 1997), conditions favored by the aforementioned pH values.
To the best of our knowledge, there is a lack of studies in the literature describing the impact of extraction conditions on the EAI of green coffee proteins, which hinders the comparison of our data with other studies. However, it is possible to infer that coffee proteins present higher emulsifying properties (especially away from the 3.0–4.0 pH range) than other plant proteins such as flaxseed, lentil, and almonds (Tirgar et al., 2017; Karaca et al., 2011; Dias & de Moura Bell, 2022), which highlights their promising use as emulsifiers in different food and cosmetic products.
Foaming Capacity and Stability
The foaming properties of proteins are frequently associated with their ability to be adsorbed onto the air/water interface and to decrease surface tension, creating a strong interfacial film via protein–protein interactions (Day, 2013). The influence of the AEP and EAEP on foaming capacity (FC) and foaming stability (FS) of green coffee protein extracts generated by the two-stage countercurrent process is shown in Fig. 6.
Effect of two-stage countercurrent AEP and EAEP on foaming capacity (FC) (A) and foaming stability (FS) evaluated at 10 min (B) for coffee proteins. Different letters in the columns indicate statistical difference (p < 0.05) among samples, independent of the extraction process (AEP vs. EAEP) and pH evaluated
FC and FS of green coffee proteins were affected by the pH, with higher values observed at lower pH values. As previously reported for amaranth proteins, good foaming properties at acidic pH can be associated with both higher protein unfolding and viscoelasticity, besides greater flexibility of the protein at acidic medium, compared with alkaline pH (Bolontrade et al., 2016). In general, coffee proteins obtained by aqueous extraction presented higher FC than EAEP proteins obtained by enzymatic extraction, with the highest value at pH 2.0 and 4.0 and the lowest value at pH 9.0 (Fig. 6A). Structural modifications arising either from pH changes or proteolysis can influence the conformation of the proteins at air/water interfaces, impacting the interfacial rheology and, consequently, the foaming properties (Day, 2013). Proteolysis reduced the surface hydrophobicity of coffee proteins (Table 4), which probably contributed to the decrease in FC of the EAEP protein extracts (statistically significant at pH 2, 4, and 7) (Fig. 6A). Others have reported that reduced H0 due to proteolysis exposes negative charges on the structure of smaller peptides, preventing the formation of stable foams (Achouri et al., 1998).
FS followed a similar trend to FC when evaluated at 10 min, with higher FS (117%) observed for AEP skim proteins at acid pH (2.0), followed by the pH 3.0–4.0 (46.7–72.5%) (Fig. 6B). The higher FS skim proteins at pH values close to the pI could be attributed to the higher electrostatic attraction between proteins, which can adsorb at the interface and decrease the interfacial tension forming a rigid, viscous, and elastic film (Zayas, 1997).
Foaming stability after 30 and 60 min was also evaluated (Fig. 7) and the foams were stable at most pH values, except at pH 2.0, which presented an expressive decay for both AEP (from 117 to 31%, when evaluated at 10 and 60 min, respectively) and EAEP proteins (from 84.5 to 0%, when evaluated at 10 and 60 min, respectively). Thus, considering FS at longer periods, coffee protein foams present poor stability at pH 2.0 and higher stability at the pH range from 3.0 to 4.0.
In general, AEP foams presented higher FS over time when compared to EAEP foams (Fig. 7), which could be related to the skim compositions, where AEP skims presented lower oil (2.41 vs 3.84%, woil/wdried skim) and higher carbohydrate content (60.8 vs. 56.9%, wcarbohydrates/wdried skim) (Table 3). It is known that coffee oil can act as an antifoam agent due to its ability to retard the foam-forming and stabilizing activities through the mechanisms of bubble breakage and shrinkage (Padma Ishwarya & Nisha, 2021). Furthermore, coffee carbohydrates can increase the viscosity of the solution and favor the protein film thickness at the interface acting as foam stabilizers (Arya & Rao, 2007), therefore justifying the better FS of the foams prepared with AEP skims.
Conclusions
The findings present herein highlight, for the first time, the influence of extraction conditions on the extractability and structural modifications altering the functionality of green coffee proteins and the integrated impact of extraction conditions and cream demulsification strategies on the recovery of the extracted oil. Oil and protein extraction yields of 58 and 72% were achieved at the 1.14 kg scale, respectively; being similar to the ones obtained at the 0.05 kg scale. The use of enzyme during the multistage extraction, besides increasing protein extraction yields by 13%, compared to the aqueous extraction process, resulted in modifications in the physicochemical characteristics (e.g., reduced size and surface hydrophobicity) of the extracted protein. Although proteolysis resulted in smaller and more soluble protein fragments/peptides at acidic pH (3.0–5.0), it did not significantly alter their emulsifying properties, except at pH 3.5, where AEP proteins had a significantly higher emulsification activity index. Overall, the emulsification properties of AEP and EAEP green coffee proteins were high, despite the extraction methods used. Nonetheless, proteolysis significantly reduced the foaming capacity and stability of the hydrolysates at some of the pH values evaluated. Importantly, enzymatic extraction produced an emulsion with reduced stability to both chemical and enzymatic demulsification approaches. Our study highlights that the two-stage countercurrent AEP/EAEP are effective environmentally-friendly processing strategies for the production of green coffee lipids and proteins with desired functional properties.
Data Availability
All data generated or analyzed during this study are included in this manuscript.
References
Achouri, A., Zhang, W., & Shiying, X. (1998). Enzymatic hydrolysis of soy protein isolate and effect of succinylation on the functional properties of resulting protein hydrolysates. Food Research International, 31(9), 617–623. https://doi.org/10.1016/S0963-9969(98)00104-5
AOAC. (1990). Official Methods Analysis (15th ed.). AOAC.
Applied Food Sciences, Inc. (2019). Everybody loves coffee, what about coffee protein? https://www.globenewswire.com/newsrelease/2019/10/15/1929930/0/en/Everybody-Loves-Coffee-What-About-Coffee-Protein.html. Accessed 28 Jan 22.
Arya, M., & Rao, L. J. M. (2007). An impression of coffee carbohydrates. Critical Reviews in Food Science and Nutrition, 47(1), 51–67. https://doi.org/10.1080/10408390600550315
Bolontrade, A. J., Scilingo, A. A., & Añón, M. C. (2016). Amaranth proteins foaming properties: Film rheology and foam stability - Part 2. Colloids and Surfaces B: Biointerfaces, 141, 643–650. https://doi.org/10.1016/j.colsurfb.2014.10.061
Bornhorst, G. M., & Singh, R. P. (2013). Kinetics of in vitro bread bolus digestion with varying oral and gastric digestion parameters. Food Biophysics, 8(1), 50–59. https://doi.org/10.1007/s11483-013-9283-6
Cathy Siegner. (2019). Will coffee bean protein get consumers buzzing? https://www.fooddive.com/news/will-coffee-bean-protein-get-consumers-buzzing/565812/. Accessed 28 Jan 2022.
Chabrand, R. M., & Glatz, C. E. (2009). Destabilization of the emulsion formed during the enzyme-assisted aqueous extraction of oil from soybean flour. Enzyme and Microbial Technology, 45(1), 28–35. https://doi.org/10.1016/j.enzmictec.2009.03.008
Chemat, F., Vian, M. A., Ravi, H. K., Khadhraoui, B., Hilali, S., Perino, S., & Fabiano Tixier, A.-S. (2019). Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules, 24, 3007. https://doi.org/10.3390/molecules24163007
Damodaran, S., Parkin, K. L., & Fennema, O. R. (2008). Fennema’s Food Chemistry (4th ed.). CRC Press/Taylor & Francis.
Day, L. (2013). Proteins from land plants–Potential resources for human nutrition and food security. Trends in Food Science and Technology, 32(1), 25–42. https://doi.org/10.1016/j.tifs.2013.05.005
De Almeida, N. M., De Moura Bell, J. M. L. N., & Johnson, L. A. (2014). Properties of soy protein produced by countercurrent two-stage enzyme-assisted aqueous extraction. JAOCS, Journal of the American Oil Chemists’ Society, 91(6), 1077–1085. https://doi.org/10.1007/s11746-014-2436-z
de Aquino, D. S., Fanhani, A., Stevanato, N., & da Silva, C. (2019). Sunflower oil from enzymatic aqueous extraction process: Maximization of free oil yield and oil characterization. Journal of Food Process Engineering, 42(6), 1–10. https://doi.org/10.1111/jfpe.13169
De Moura, J. M. L. N., De Almeida, N. M., & Johnson, L. A. (2009). Scale-up of enzyme-assisted aqueous extraction processing of soybeans. JAOCS, Journal of the American Oil Chemists’ Society, 86(8), 809–815. https://doi.org/10.1007/s11746-009-1406-3
De Moura, J. M. L. N., Maurer, D., Jung, S., & Johnson, L. A. (2011). Integrated countercurrent two-stage extraction and cream demulsification in enzyme-assisted aqueous extraction of soybeans. JAOCS, Journal of the American Oil Chemists’ Society, 88(7), 1045–1051. https://doi.org/10.1007/s11746-011-1759-2
de Souza, T. S. P., Dias, F. F. G., Koblitz, M. G. B., & de Moura Bell, J. M. L. N. (2020a). Effects of enzymatic extraction of oil and protein from almond cake on the physicochemical and functional properties of protein extracts. Food and Bioproducts Processing, 122, 280–290. https://doi.org/10.1016/j.fbp.2020.06.002
de Souza, T. S. P., Dias, F. F. G., Oliveira, J. P. S., de Moura Bell, J. M. L. N., & Koblitz, M. G. B. (2020b). Biological properties of almond proteins produced by aqueous and enzyme-assisted aqueous extraction processes from almond cake. Scientific Reports, 10(1), 1–12. https://doi.org/10.1038/s41598-020-67682-3
Dias, F. F. G., de Almeida, N. M., de Souza, T. S. P., Taha, A. Y., Juliana, M., & Bell, L. N. D. M. (2020). Characterization and demulsification of the oil-rich emulsion from the aqueous extraction process of almond flour. Processes, 8(10), 1228. https://doi.org/10.3390/pr8101228
Dias, F. F. G., & de Moura Bell, J. M. L. N. (2022). Understanding the impact of enzyme-assisted aqueous extraction on the structural, physicochemical, and functional properties of protein extracts from full-fat almond flour. Food Hydrocolloids, 127, 107534. https://doi.org/10.1016/j.foodhyd.2022.107534
Goertzen, A. D., House, J. D., Nickerson, M. T., & Tanaka, T. (2021). The impact of enzymatic hydrolysis using three enzymes on the nutritional properties of a chickpea protein isolate. Cereal Chemistry, 98(2), 275–284. https://doi.org/10.1002/cche.10361
Jakubczyk, A., Świeca, M., Gawlik-Dziki, U., & Dziki, D. (2018). Nutritional potential and inhibitory activity of bread fortified with green coffee beans against enzymes involved in metabolic syndrome pathogenesis. LWT-Food Science and Technology, 95, 78–84. https://doi.org/10.1016/j.lwt.2018.04.071
Jamdar, S. N., Rajalakshmi, V., Pednekar, M. D., Juan, F., Yardi, V., & Sharma, A. (2010). Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chemistry, 121(1), 178–184. https://doi.org/10.1016/j.foodchem.2009.12.027
Jiang, L., Hua, D., Wang, Z., & Xu, S. (2010). Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food and Bioproducts Processing, 88(2–3), 233–238. https://doi.org/10.1016/j.fbp.2009.08.002
Jiang, L., Wang, Z., Li, Y., Meng, X., Sui, X., Qi, B., & Zhou, L. (2015). Relationship between surface hydrophobicity and structure of soy protein isolate subjected to different ionic strength. International Journal of Food Properties, 18(5), 1059–1074. https://doi.org/10.1080/10942912.2013.865057
Jung, S., Maurer, D., & Johnson, L. A. (2009). Factors affecting emulsion stability and quality of oil recovered from enzyme-assisted aqueous extraction of soybeans. Bioresource Technology, 100(21), 5340–5347. https://doi.org/10.1016/j.biortech.2009.03.087
Karaca, A. C., Low, N., & Nickerson, M. (2011). Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Research International, 44(9), 2742–2750. https://doi.org/10.1016/j.foodres.2011.06.012
Laguna, L., Picouet, P., Guàrdia, M. D., Renard, C. M. G. C., & Sarkar, A. (2017). In vitro gastrointestinal digestion of pea protein isolate as a function of pH, food matrices, autoclaving, high-pressure and re-heat treatments. LWT-Food Science and Technology, 84, 511–519. https://doi.org/10.1016/j.lwt.2017.06.021
Lam, R. S. H., & Nickerson, M. T. (2013). Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chemistry, 141(2), 975–984. https://doi.org/10.1016/j.foodchem.2013.04.038
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Mat Yusoff, M., Gordon, M. H., & Niranjan, K. (2015). Aqueous enzyme assisted oil extraction from oilseeds and emulsion de-emulsifying methods: A review. Trends in Food Science and Technology, 41(1), 60–82. https://doi.org/10.1016/j.tifs.2014.09.003
Mechqoq, H., el Yaagoubi, M., Momchilova, S., Msanda, F., & el Aouad, N. (2021). Comparative study on yields and quality parameters of argan oils extracted by conventional and green extraction techniques. Grain & Oil Science and Technology, 4(3), 125–130. https://doi.org/10.1016/J.GAOST.2021.08.002
Nielsen, P. M., Petersen, D., & Dambmann, C. (2001). Improved method for determining food protein degree of hydrolysis. Food Chemistry and Toxicology, 66(5), 642–646. https://doi.org/10.1111/j.1365-2621.2001.tb04614.x
Oliveira, R., Carvalho, G. R., Cirillo, M., & Queiroz, F. (2019b). Effect of ecofriendly bio-based solvents on oil extraction from green coffee bean and its industrial press cake. Brazilian Journal of Chemical Engineering, 36(4), 1739–1753. https://doi.org/10.1590/0104-6632.20190364s20190102
Oliveira, É. R., Silva, R. F., Santos, P. R., & Queiroz, F. (2019a). Potential of alternative solvents to extract biologically active compounds from green coffee beans and its residue from the oil industry. Food and Bioproducts Processing, 115, 47–58. https://doi.org/10.1016/j.fbp.2019.02.005
Padma Ishwarya, S., & Nisha, P. (2021). Foaming agents from spent coffee grounds: A mechanistic understanding of the modes of foaming and the role of coffee oil as antifoam. Food Hydrocolloids, 112, 106354. https://doi.org/10.1016/j.foodhyd.2020.106354
Pearce, K. N., & Kinsella, J. E. (1978). Emulsifying properties of proteins: Evaluation of a turbidimetric technique. Journal of Agricultural and Food Chemistry, 26(3), 716–723. https://doi.org/10.1021/jf60217a041
Pojić, M., Mišan, A., & Tiwari, B. (2018). Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends in Food Science and Technology, 75, 93–104. https://doi.org/10.1016/j.tifs.2018.03.010
Prandi, B., Ferri, M., Monari, S., Zurlini, C., Cigognini, I., Verstringe, S., Schaller, D., Walter, M., Navarini, L., Tassoni, A., Sforza, S., & Tedeschi, T. (2021). Extraction and chemical characterization of functional phenols and proteins from coffee (Coffea arabica) by-products. Biomolecules, 11(11), 1571. https://doi.org/10.3390/biom11111571
Polmann, G., Badia, V., Frena, M., Teixeira, G. L., Rigo, E., Block, J. M., & Camino Feltes, M. M. (2019). Enzyme-assisted aqueous extraction combined with experimental designs allow the obtaining of a high-quality and yield pecan nut oil. LWT- Food Science and Technology, 113, 108283. https://doi.org/10.1016/J.LWT.2019.108283
Redgwell, R., & Fischer, M. (2006). Coffee carbohydrates. Brazilian Journal of Plant Physiology, 18(1), 165–174. https://doi.org/10.1590/S1677-04202006000100012
Ricardo, F., Pradilla, D., Cruz, J. C., & Alvarez, O. (2021). Emerging emulsifiers: Conceptual basis for the identification and rational design of peptides with surface activity. International Journal of Molecular Sciences, 22, 4615. https://doi.org/10.3390/ijms22094615
Soto, C., Chamy, R., & Zúñiga, M. E. (2007). Enzymatic hydrolysis and pressing conditions effect on borage oil extraction by cold pressing. Food Chemistry, 102(3), 834–840. https://doi.org/10.1016/J.FOODCHEM.2006.06.014
Souza, T. S. P., Dias, F. F. G., Koblitz, M. G. B., & De Moura Bell, J. M. L. N. (2019). Aqueous and enzymatic extraction of oil and protein from almond cake: A comparative study. Processes, 7(7), 472. https://doi.org/10.3390/pr7070472
Souza Almeida, F., Furlan Goncalves Dias, F., Kawazoe Sato, A. C., & Leite Nobrega de Moura Bell, J. M. (2021). From solvent extraction to the concurrent extraction of lipids and proteins from green coffee: An eco-friendly approach to improve process feasibility. Food and Bioproducts Processing, 129, 144–156. https://doi.org/10.1016/j.fbp.2021.08.004
Tabtabaei, S., & Diosady, L. L. (2013). Aqueous and enzymatic extraction processes for the production of food-grade proteins and industrial oil from dehulled yellow mustard flour. Food Research International, 52(2), 547–556. https://doi.org/10.1016/j.foodres.2013.03.005
Thamnarathip, P., Jangchud, K., Jangchud, A., & Vardhanabhuti, B. (2016). Functional properties of protein hydrolysates from Riceberry rice bran. International Journal of Food Science and Technology, 51(5), 1110–1119. https://doi.org/10.1111/ijfs.13071
Tirgar, M., Silcock, P., Carne, A., & Birch, E. J. (2017). Effect of extraction method on functional properties of flaxseed protein concentrates. Food Chemistry, 215, 417–424. https://doi.org/10.1016/j.foodchem.2016.08.002
Voytena, A. P. L., Affonso, R. C. L., da Silva Pitz, H., Ramlov, F., Alberti, T., Coelho, D. S., Pereira, A., Navarro, B. B., Fanan, S., Casagrande, M., Ribeiro-do-Valle, R. M., & Maraschin, M. (2017). Phytochemical profile and in vitro assessment of the cytotoxicity of green and roasted coffee oils (Coffea arabica L.) and their polar fractions. Records of Natural Products, 12(2), 169–174. https://doi.org/10.25135/rnp.18.17.06.108
Wen, L., Álvarez, C., Zhang, Z., Poojary, M. M., Lund, M. N., Sun, D. W., & Tiwari, B. K. (2021). Optimisation and characterisation of protein extraction from coffee silverskin assisted by ultrasound or microwave techniques. Biomass Conversion and Biorefinery, 11, 1575–15785. https://doi.org/10.1007/s13399-020-00712-2
Wu, W. U., Hettiarachchy, N. S., & Qi, M. (1998). Hydrophobicity, solubility, and emulsifying properties of soy protein peptides prepared by papain modification and ultrafiltration. JAOCS, Journal of the American Oil Chemists’ Society, 75(7), 845–850. https://doi.org/10.1007/s11746-998-0235-0
Wu, J., Johnson, L. A., & Jung, S. (2009). Demulsification of oil-rich emulsion from enzyme-assisted aqueous extraction of extruded soybean flakes. Bioresource Technology, 100(2), 527–533. https://doi.org/10.1016/j.biortech.2008.05.057
Zayas, J. F. (1997). Functionality of Proteins in Food. Springer.
Zhang, S. B., Liu, X. J., Lu, Q. Y., Wang, Z. W., & Zhao, X. (2013a). Enzymatic demulsification of the oil-rich emulsion obtained by aqueous extraction of peanut seeds. JAOCS, Journal of the American Oil Chemists’ Society, 90(8), 1261–1270. https://doi.org/10.1007/s11746-013-2265-5
Zhang, Y., Zhao, W., Yang, R., Ahmed, M. A., Hua, X., Zhang, W., & Zhang, Y. (2013b). Preparation and functional properties of protein from heat-denatured soybean meal assisted by steam flash-explosion with dilute acid soaking. Journal of Food Engineering, 119(1), 56–64. https://doi.org/10.1016/j.jfoodeng.2013.05.008
Funding
Funding for this study was made possible by the USDA National Institute of Food and Agriculture, Hatch/Multi State project [1023517] and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Brazil (Finance code 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-141112/2018–2) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #2018/21987–1 and #2019/27354–3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almeida, F.S., Dias, F.F.G., Sato, A.C.K. et al. Scaling up the Two-Stage Countercurrent Extraction of Oil and Protein from Green Coffee Beans: Impact of Proteolysis on Extractability, Protein Functionality, and Oil Recovery. Food Bioprocess Technol 15, 1794–1809 (2022). https://doi.org/10.1007/s11947-022-02831-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02831-2