Abstract
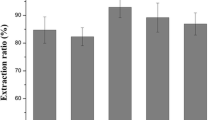
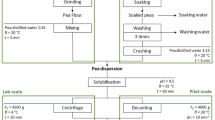
Papaya peel is a good source of papain enzyme (cysteine protease) but currently unutilized for any commercial purpose and disposed of as waste, thereby becoming a source of pollution. Therefore, this study investigates the behavior of papain enzyme (cysteine protease) in an aqueous two-phase system (ATPS) comprising polymer/salt. Considering the excluded volume effect, polyethylene glycol (PEG) with a molecular weight of 6000 g/mol was used to form the ATPS. Sulphate salts (ammonium sulphate and sodium sulphate) were chosen to form phases owing to their ability to promote the hydrophobic difference between the phases. From the result, the best ATPS condition for the partitioning of cysteine protease was 10% (w/w) PEG 6000 + 18% (w/w) Na2SO4 at constant pH 9 and obtained the highest enzyme activity (Ke) 1.43 which increased the purity by 4.08-fold (PF) with the yield of 26.38%. PEG/salt ATPS has been shown to work well to purify partitioned cysteine protease, which can be scaled up and extracted with a high yield and purity factor. In addition, the paper studies the (Sodium dodecyl-sulfate polyacrylamide gel electrophoresis) SDS-PAGE patterns of cysteine protease partitioned from papaya peels. The ATPS system is therefore effective in recovering and purifying papain enzymes from papaya peel.

Similar content being viewed by others
Data availability
Data will be made available on request.
References
W. Aehle, Enzymes in Industry: Production and Application (Wiley-VCH, New York, 2004)
R. Tomar, R. Kumar, M.V. Jagannadham, J. Agric, Food Chem. 56(4), 1479–1487 (2008). https://doi.org/10.1021/jf0726536
A. Sumantha, C. Larroche, A. Pandey, Food Technol. Biotechnol. 44, 211–220 (2006)
M. Singla, A. Singh, N. Sit, J. Food Process. Eng. e14119 (2022). https://doi.org/10.1111/jfpe.4119
V. Yogiraj, P.K. Goyal, C.S. Chauhan, A. Goyal, B. Vyas, Int. J. Herb. Med. 2(5), 01–08 (2014)
FAOSTAT (2021). http://www.fao.org/faostat/en/#data/QC
P.D. Pathak, S.A. Mandavgane, B.D. Kulkarni, Waste Biomass Valorization 10(6), 1755–1766 (2019). https://doi.org/10.1007/s12649-017-0181-x
Y.Q. Ling, H.L. Nie, S.N. Su, C. Branford-White, L.M. Zhu, Sep. Purif. Technol. 73(3), 343–348 (2010). https://doi.org/10.1016/j.seppur.2010.04.020
M. Zhang, P. Hu, Q. Liang, H. Yang, Q. Liu, Y. Wang, G. Luo, J. Chromatogr. B 858(1–2), 220–226 (2007). https://doi.org/10.1016/j.jchromb.2007.08.041
R. Gupta, Q. Beg, P. Lorenz, Appl. Microbiol. Biotechnol. 59(1), 15–32 (2002). https://doi.org/10.1007/s00253-002-0975-y
J.P. Chen, M.S. Lee, Enzyme Microb. Technol. 17(11), 1021–1027 (1995). https://doi.org/10.1016/0141-0229(95)00030-5
E. Andersson, B. Hahn-Hägerdal, Enzyme Microb. Technol. 12(4), 242–254 (1990). https://doi.org/10.1016/0141-0229(90)90095-8
T. Tianwei, H. Qing, L. Qiang, Biotechnol. Lett. 24(17), 1417–1420 (2002). https://doi.org/10.1023/A:1019850531640
R. Hatti-Kaul, Mol. Biotechnol. 19(3), 269–277 (2001). https://doi.org/10.1385/MB:19:3:269
H. Hustedt, K.H. Kroner, N. Papamichael, Process. Biochem. 23(5), 129–137 (1988)
A. Veide, A.L. Smeds, S.O. Enfors, Biotechnol. Bioeng. 25(7), 1789–1800 (1983). https://doi.org/10.1002/bit.260250709
M.R. Kula, K.H. Kroner, H. Hustedt, H. Schutte, Enzym Eng. 69–74 (1982). https://doi.org/10.1007/978-1-4615-9290-7_6
U. Menge, in Aqueous two-phase systems: methods and protocols, ed. by R. Hatti-Kaul (Humana Press, Totowa, 2000) pp. 235–249
P. Albertsson, Partition of cell Particles and Macromolecules, 3rd edn. (Wiley, New York, 1986)
A. Venâncio, C. Almeida, J. Teixeira, J. Chromatogr, B Biomed. Appl. 680(1–2), 131–136 (1996). https://doi.org/10.1016/0378-4347(95)00419-X
H. Hustedt, K.H. Kroner, M.R. Kula, in Partitioning in Aqueous Two-Phase Systems; Theory, Methods, Uses and Application in Biotechnology, ed. by H. Walter, D. E. Brooks, D. Fisher, (Academic press, Orlando, 1985) pp. 529–587
J. Vernau, M.R. Kula, Biotechnol. Appl. Biochem. 12(4), 397–404 (1990)
M.M. Bradford, Anal. Biochem. 72(1–2), 248–254 (1976). https://doi.org/10.1006/abio.1976.9999
U.K. Laemmli, Nature 227, 680–685 (1970). https://doi.org/10.1038/227680a0
A.L. Grilo, M. Raquel Aires-Barros, A.M. Azevedo, Sep. Purif. Rev. 45(1), 68–80 (2016). https://doi.org/10.1080/15422119.2014.983128
E.L. Smith, J.R. Kimmel, D.M. Brown, J. Biol. Chem. 207(2), 533–549 (1954). https://doi.org/10.1016/S0021-9258(18)65670-4
J.A. Asenjo, B.A. Andrews, J. Chromatogr. A 1218(49), 8826–8835 (2011). https://doi.org/10.1016/j.chroma.2011.06.051
J.C. Marcos, L.P. Fonseca, M.T. Ramalho, J.M.S. Cabral, J. Chromatogr, B Biomed. Appl. B 711, 295–299 (1998). https://doi.org/10.1016/S0378-4347(97)00633-6
M.A. Eiteman, J.L. Gainer, Biochim. Biophys. Acta Gen. Subj. 1073(3), 451–455 (1991). https://doi.org/10.1016/0304-4165(91)90214-2
T. Arakawa, L.O. Narhi, Biotechnol. Appl. Biochem. 13(2), 151–172 (1991)
T. Arakawa, S.N. Timasheff, Biochemistry 23(25), 5912–5923 (1984). https://doi.org/10.1021/bi00320a004
B.Y. Zaslavsky, Aqueous two-phase Partitioning: Physical Chemistry and Bioanalytical Applications (CRC press,, New York, 1994)
A. Salabat, M.H. Abnosi, A.R. Bahar, J. Chromatogr. B 858, 234–238 (2007). https://doi.org/10.1016/j.jchromb.2007.08.039
H. Yue, Q. Yuan, W. Wang, Biochem. Eng. J. 37(3), 231–237 (2007). https://doi.org/10.1016/j.bej.2007.05.002
G. Johansson, Mol. Cell. Biochem. 4(3), 169–180 (1974). https://doi.org/10.1007/BF01731478
S. Nalinanon, S. Benjakul, W. Visessanguan, H. Kishimura, Process. Biochem. 44(4), 471–476 (2009). https://doi.org/10.1016/j.procbio.2008.12.018
R. Tapadar, M. Nandi, R. Chatterjee, K. Mukherjee, K. Majumder, J. BioSci. Biotechnol. 8(2), 168–173 (2019)
M. de H.C. Maciel, C.A. Ottoni, P.N. Herculano, T.S. Porto, A.L. Porto, C. Santos, L. Nelson, A.M. Keila, C. Souza-Motta, Fluid Ph. Equilibria 371, 125–130 (2014). https://doi.org/10.1016/j.fluid.2014.03.018
M.A. Fakhari, F. Rahimpour, M. Taran, J. Chromatogr. B 1063, 1–10 (2017). https://doi.org/10.1016/j.jchromb.2017.08.007
K. Naganagouda, V.H. Mulimani, Process. Biochem. 43(11), 1293–1299 (2008). https://doi.org/10.1016/j.procbio.2008.07.016
J.N. Baskir, T.A. Hatton, U.W. Suter, Biotechnol. Bioeng. 34(4), 541–558 (1989). https://doi.org/10.1002/bit.260340414
T. Vernet, P.J. Berti, C. de Montigny, R. Musil, D.C. Tessier, R. Ménard, M.C. Magny, A.C. Storer, D.Y. Thomas, J. Biol. Chem. 270(18), 10838–10846 (1995). https://doi.org/10.1074/jbc.270.18.10838
Acknowledgements
The authors would like to acknowledge the fellowship received from All India Council of Technical Education (AICTE), New Delhi, India for carrying out the work. The authors would also like to acknowledge the help received from Prof. Robin Doley and Ms. Nyumpi Bagra for using the facilities of their lab and helping in analysis of the samples.
Funding
No external funding was received to carry out the present work.
Author information
Authors and Affiliations
Contributions
Mohit Singla: Methodology, validation, Formal analysis, investigation, Writing-original draft; Nandan Sit: Conceptualization, validation, writing-review and editing, visualization, supervision, project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singla, M., Sit, N. Isolation of papain from ripe papaya peel using aqueous two-phase extraction. Food Measure 17, 1685–1692 (2023). https://doi.org/10.1007/s11694-022-01741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01741-3