Abstract
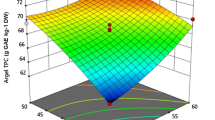
Response surface methodology (RSM) and a Box-Behnken design (BBD) were used to determine optimum conditions for ultrasound-assisted extraction (UAE) of Hibiscus sabdariffa L. calyces. The current study applied BBD to explore the effects of X1: ultrasonic temperature (30–80 °C), X2: ultrasonic time (20–50 min.), and X3: solid-to-solvent ratio (1:10–1:60) on total anthocyanin content (TAC), total phenolic content (TPC), and antioxidant activities (2,2-diphenylpicrylhydrazyl (DPPH) and ferric ion reducing antioxidant power (FRAP) assays). ANOVA results revealed that TAC, TPC, DPPH, and FRAP all had R2 values of 0.98, 0.97, 0.98, and 0.98, respectively, indicating that models designed with second-order polynomials were capable of reliably analyzing interactions between parameters (response and independent variables) satisfactorily. It was determined from the RSM study that 80 °C ultrasound temperature, 48 min. ultrasound time, and 1:60 solid-to-solvent ratio were the optimum extraction parameters for maximizing TAC, TPC, DPPH, and FRAP. The experimental values for TAC, TPC, DPPH, and FRAP were 311 ± 5 mg CGE/100 g, 572 ± 7 mg GAE/100 g, 974 ± 3 μmolTE/100 mL, and 2332 ± 3 μmolTE/100 mL, respectively, under the optimal conditions. Also, a good agreement was found between experimental and predicted values, with a residual standard error of less than 5%. Compared to the yield of Soxhlet extraction for TAC (176 ± 4 mg CGE/100 g), TPC (210 ± 3 mg GAE/100 g), DPPH (534 ± 2 μmolTE/100 mL), and FRAP (1732 ± 3 μmolTE/100 mL), the extraction efficacy of the UAE process under optimized conditions demonstrated to be more effective. Therefore, based on the needs of the industry and sustainable development, the UAE process might be an economical alternative to traditional extraction methods.





Similar content being viewed by others
Data availability
Not applicable.
References
Galanakis CM (2021) Functionality of food components and emerging technologies. Foods 10:128
Salem MA, Zayed A, Beshay ME, Abdel Mesih MM, Ben Khayal RF, George FA, Ezzat SM (2021) Hibiscus sabdariffa L.: phytoconstituents, nutritive, and pharmacological applications. Adv Tradit Med:1–11
Prasongwatana V, Woottisin S, Sriboonlue P, Kukongviriyapan V (2008) Uricosuric effect of Roselle (Hibiscus sabdariffa) in normal and renal-stone former subjects. J Ethnopharmacol 117:491–495
Olvera‐García V, Castaño‐Tostado E, Rezendiz‐Lopez RI, Reynoso‐Camacho R, González de Mejía E, Elizondo G, Loarca‐Piña G (2008) Hibiscus sabdariffa L. extracts inhibit the mutagenicity in microsuspension assay and the proliferation of HeLa cells. J Food Sci 73:T75–T81
Wong-Paz JE, Muñiz-Márquez DB, Aguilar-Zárate P, Ascacio-Valdés JA, Cruz K, Reyes-Luna C, Rodríguez R, Aguilar CN (2017) Extraction of bioactive phenolic compounds by alternative technologies. Ingredients Extr by Physicochem methods food Elsevier:229–252
Picot-Allain C, Mahomoodally MF, Ak G, Zengin G (2021) Conventional versus green extraction techniques—a comparative perspective. Curr Opin Food Sci 40:144–156
Kumar K, Srivastav S, Sharanagat VS (2021) Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason Sonochem 70:105325
Usman I, Hussain M, Imran A, Afzaal M, Saeed F, Javed M, Afzal A, Ashfaq I, Al Jbawi E, A Saewan S (2022) Traditional and innovative approaches for the extraction of bioactive compounds. Int J Food Prop 25:1215–1233. https://doi.org/10.1080/10942912.2022.2074030
Ou-Ani O, Oucheikh L, Dabbous A, Znini M, Costa J, Majidi L (2022) Optimization of hydrodistillation extraction using response surface methodology and chemical composition of essential oil from Moroccan endemic medicinal plant Ballota hirsuta, vol 4
Breig SJM, Luti KJK (2021) Response surface methodology: a review on its applications and challenges in microbial cultures. Mater Today Proc 42:2277–2284
Ahmed T, Rana MR, Maisha MR, Sayem ASM, Rahman M, Ara R (2022) Optimization of ultrasound-assisted extraction of phenolic content & antioxidant activity of hog plum (Spondias pinnata L. f. kurz) pulp by response surface methodology. Heliyon:131932. https://doi.org/10.1016/j.heliyon.2022.e11109
Borges GDSC, Vieira FGK, Copetti C, Gonzaga LV, Fett R (2011) Optimization of the extraction of flavanols and anthocyanins from the fruit pulp of Euterpe edulis using the response surface methodology. Food Res Int 44:708–715. https://doi.org/10.1016/j.foodres.2010.12.025
Kothari V, Gupta A, Naraniwal M (2012) Comparative study of various methods for extraction of antioxidant and antibacterial compounds from plant seeds. J Nat Remedies 12:162–173
Lee J, Durst ROBERT, Wrolstad RONALD (2005) AOAC official method 2005.02: total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method. Off Methods Anal AOAC Int 2
Silva TLL, Silva EPD, Asquieri ER, Vieira ECS, Silva JS, Silva FAD, Damiani C (2018) Physicochemical characterization and behavior of biocompounds of caja-manga fruit (Spondias mombin L.). Food. Sci Technol 38:399–406. https://doi.org/10.1590/fst.03717
Silva EM, Souza JNS, Rogez H, Rees JF, Larondelle Y (2007) Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem 101:1012–1018. https://doi.org/10.1016/j.foodchem.2006.02.055
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH (2006) Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal 19:669–675. https://doi.org/10.1016/j.jfca.2006.01.003
Borrás-Enríquez AJ, Reyes-Ventura E, Villanueva-Rodríguez SJ, Moreno-Vilet L (2021) Effect of ultrasound-assisted extraction parameters on total polyphenols and its antioxidant activity from mango residues (Mangifera indica L). Separations 8:94
Corrales M, Toepfl S, Butz P, Knorr D, Tauscher B (2008) Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innov Food Sci & Emerg Technol 9:85–91
González-Centeno MR, Knoerzer K, Sabarez H, Simal S, Rosselló C, Femenia A (2014) Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)--a response surface approach. Ultrason Sonochem 21:2176–2184
Xu Y, Pan S (2013) Effects of various factors of ultrasonic treatment on the extraction yield of all-trans-lycopene from red grapefruit (citrus paradise Macf.). Ultrason Sonochem 20:1026–1032. https://doi.org/10.1016/j.ultsonch.2013.01.006
Dahmoune F, Boulekbache L, Moussi K, Aoun O, Spigno G, Madani K (2013) Valorization of citrus limon residues for the recovery of antioxidants: evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind Crops Prod 50:77–87. https://doi.org/10.1016/j.indcrop.2013.07.013
Carrera C, Ruiz-Rodríguez A, Palma M, Barroso CG (2012) Ultrasound assisted extraction of phenolic compounds from grapes. Anal Chim Acta 732:100–104. https://doi.org/10.1016/j.aca.2011.11.032
Leong T, Ashokkumar M, Kentish S (2011) The fundamentals of power ultrasound—a review. Acoust Aust 39:54–63
Al-Dhabi NA, Ponmurugan K, Jeganathan PM (2017) Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason Sonochem 34:206–213. https://doi.org/10.1016/j.ultsonch.2016.05.005
Samaram S, Mirhosseini H, Tan CP, Ghazali HM, Bordbar S, Serjouie A (2015) Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem 172:7–17
Dezhkunov NV, Francescutto A, Ciuti P, Sturman F (2005) Temperature dependence of cavitation activity at different ultrasound intensity. XVI Sess Russ Acoust Soc 9:74–77
Rodrigues S, Fernandes FA, de Brito ES, Sousa AD, Narain N (2015) Ultrasound extraction of phenolics and anthocyanins from jabuticaba peel. Ind Crops Prod 69:400–407. https://doi.org/10.1016/j.indcrop.2015.02.059
Raspe DT, Ciotta SR, Zorzenon MRT, Dacome AS, da Silva C, Milani PG, da Costa SC (2021) Ultrasound-assisted extraction of compounds from Stevia leaf pretreated with ethanol. Ind Crops Prod 172:114035. https://doi.org/10.1016/j.indcrop.2021.114035
Pinela J, Prieto MA, Pereira E, Jabeur I, Barreiro MF, Barros L, Ferreira IC (2019) Optimization of heat-and ultrasound-assisted extraction of anthocyanins from Hibiscus sabdariffa calyces for natural food colorants. Food Chem 275:309–321
Vázquez-Sánchez AY, Aguilar-Zárate P, Muñiz-Márquez DB, Wong-Paz JE, Rojas R, Ascacio-Valdés JA, Martínez-Ávila GCG (2019) Effect of ultrasound treatment on the extraction of antioxidants from Ardisia compressa Kunth fruits and identification of phytochemicals by HPLC-ESI-MS. Heliyon 5(12):e03058. https://doi.org/10.1016/j.heliyon.2019.e03058
Izadiyan P, Hemmateenejad B (2016) Multi-response optimization of factors affecting ultrasonic assisted extraction from Iranian basil using central composite design. Food Chem 190:864–870. https://doi.org/10.1016/j.foodchem.2015.06.036
Liu Y, She XR, Huang JB, Liu MC, Zhan ME (2017) Ultrasonic-extraction of phenolic compounds from Phyllanthus urinaria: optimization model and antioxidant activity. Food Sci Technol 38:286–293. https://doi.org/10.1590/1678-457X.21617
Xu J, Wang W, Liang H, Zhang Q, Li Q (2015) Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma longa L. using response surface methodology. Ind Crops Prod 76:487–493
Hossain MA, Ahmed T, Hossain MS, Dey P, Ahmed S, Hossain MM (2022) Optimization of the factors affecting BT-2 black tea fermentation by observing their combined effects on the quality parameters of made tea using response surface methodology (RSM). Heliyon 8:e08948. https://doi.org/10.1016/j.heliyon.2022.e08948
Himel MAR, Ahmed T, Hossain MA, Moazzem MS (2022) Response surface optimization to extract antioxidants from freeze-dried seeds and peel of pomegranate (Punica granatum L.). Biomass Convers Biorefinery 3:1–16
Kechinski CP, Guimarães PVR, Noreña CPZ, Tessaro IC, Marczak LDF (2010) Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J Food Sci 75:C173–C176
Buckow R, Kastell A, Terefe NS, Versteeg C (2010) Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. J Agric Food Chem 58:10076–10084
Barba FJ, Brianceau S, Turk M, Boussetta N, Vorobiev E (2015) Effect of alternative physical treatments (ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioprocess Technol 8:1139–1148
Barba FJ, Galanakis CM, Esteve MJ, Frigola A, Vorobiev E (2015) Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries. J Food Eng 167:38–44
Wang W, Jung J, Tomasino E, Zhao Y (2016) Optimization of solvent and ultrasound-assisted extraction for different anthocyanin rich fruit and their effects on anthocyanin compositions. LWT-Food Sci Technol 72:229–238
Zhu Z, Guan Q, Guo Y, He J, Liu G, Li S, Barba FJ, Jaffrin MY (2016) Green ultrasound-assisted extraction of anthocyanin and phenolic compounds from purple sweet potato using response surface methodology. Int Agrophysics 30
Ramić M, Vidović S, Zeković Z, Vladić J, Cvejin A, Pavlić B (2015) Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason Sonochem 23:360–368
Tiwari BK, Patras A, Brunton N, Cullen PJ, O’donnell CP (2010) Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason Sonochem 17:598–604
Golmakani MT, Moayyedi M (2016) Comparison of microwave-assisted hydrodistillation and solvent-less microwave extraction of essential oil from dry and fresh Citruslimon (Eureka variety) peel. J Essent Oil Res 28:272–282
Wang F, Zhang S, Deng G, Xu K, Xu H, Liu J (2022) Extracting total anthocyanin from purple sweet potato using an effective ultrasound-assisted compound enzymatic extraction technology. Molecules 27:4344
Ghafoor K, Choi YH, Jeon JY, Jo IH (2009) Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J Agric Food Chem 57:4988–4994
Rodrigues S, Pinto GA (2007) Ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder. J Food Eng 80:869–872
Şahin S, Şamlı R (2013) Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason Sonochem 20:595–602
Teh SS, Birch EJ (2014) Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason Sonochem 21:346–353
Vuong QV, Goldsmith CD, Dang TT, Nguyen VT, Bhuyan DJ, Sadeqzadeh E, Scarlett CJ, Bowyer MC (2014) Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant capacity from Euphorbia tirucalli using response surface methodology. Antioxidants 3:604–617
Predescu NC, Papuc C, Nicorescu V, Gajaila IULIANA, Goran GV, Petcu CD, Stefan GEORGETA (2016) The influence of solid-to-solvent ratio and extraction method on total phenolic content, flavonoid content and antioxidant properties of some ethanolic plant extracts. Rev Chim 67:1922–1927
Stamatopoulos K, Chatzilazarou A, Katsoyannos E (2013) Optimization of multistage extraction of olive leaves for recovery of phenolic compounds at moderated temperatures and short extraction times. Foods 3:66–81
Rodrigues S, Pinto GA, Fernandes FA (2008) Optimization of ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder by response surface methodology. Ultrason Sonochem 15:95–100
Sridhar A, Ponnuchamy M, Kumar PS, Kapoor A, Vo DVN, Prabhakar S (2021) Techniques and modeling of polyphenol extraction from food: a review. Environ Chem Lett 19:3409–3443
Jemain SFP, Jamal P, Raus AR, Amid A, Jaswir I (2017) Effects of process conditions on the ultrasonic extraction of phenolics scavenger from Curcuma caesia rhizome. Int Food Res J:24
Carciochi RA, Manrique GD, Dimitrov K (2015) Optimization of antioxidant phenolic compounds extraction from quinoa (Chenopodium quinoa) seeds. J Food Sci Technol 52:4396–4404
Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU, Lee SC (2004) Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J Agric Food Chem 52:3389–3393
Gogoi P, Chutia P, Singh P, Mahanta CL (2019) Effect of optimized ultrasound-assisted aqueous and ethanolic extraction of Pleurotus citrinopileatus mushroom on total phenol, flavonoids and antioxidant properties. J Food Process Eng 42:e13172
Tzima K, Brunton NP, Lyng JG, Frontuto D, Rai DK (2021) The effect of pulsed electric field as a pre-treatment step in ultrasound assisted extraction of phenolic compounds from fresh rosemary and thyme by-products. Innov Food Sci & Emerg Technol 69:102644
Hossain MA, Hossain MS (2021) Optimization of antioxidant extraction from freeze-dried pulp, peel, and seed of Burmese grape (Baccaurea ramiflora Lour.) by response surface methodology. Biomass Convers Biorefinery 6. https://doi.org/10.21203/rs.3.rs-347432/v1
Hani NM, Torkamani AE, Abidin SZ, Mahmood WAK, Juliano P (2017) The effects of ultrasound assisted extraction on antioxidative activity of polyphenolics obtained from Momordica charantia fruit using response surface approach. Food Biosci 17:7–16. https://doi.org/10.1016/j.fbio.2016.11.002
Deng GF, Xu DP, Li S, Li HB (2015) Optimization of ultrasound-assisted extraction of natural antioxidants from sugar apple (Annona squamosa L.) peel using response surface methodology. Molecules 20:20448–20459. https://doi.org/10.3390/molecules201119708
Chan SW, Lee CY, Yap CF, Wan Aida WM, Ho CW (2009) Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Int Food Res J 16
Vickers NJ (2017) Animal communication: when I’m calling you, will you answer too? Curr Biol 27:R713–R715. https://doi.org/10.1016/j.cub.2017.05.064
Chen S, Zeng Z, Hu NA, Bai BO, Wang H, Suo Y (2018) Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem 242:1–8. https://doi.org/10.1016/j.foodchem.2017.08.105
Kashyap P, Riar CS, Jindal N (2021) Optimization of ultrasound assisted extraction of polyphenols from Meghalayan cherry fruit (Prunus nepalensis) using response surface methodology (RSM) and artificial neural network (ANN) approach. J Food Meas Charact 15:119–133. https://doi.org/10.1007/s11694-020-00611-0
Derringer G, Suich R (1980) Simultaneous optimization of several response variables. J Qual Technol 12:214–219
Ciric A, Krajnc B, Heath D, Ogrinc N (2020) Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem Toxicol 135:110976
Duy NQ, Thoai H, Lam T (2019) Effects of different extraction solvent systems on total phenolic, total flavonoid, total anthocyanin contents and antioxidant activities of Roselle (Hibiscus sabdariffa L.) extracts. Asian J Chem 31:2517–2521
Jafarian S, Mortazavi A, Kenari RS, RadA-HE, (2014) Total phenolic content & antioxidant activity of Roselle (Hibiscus sabdariffa L.) calyces extracts. J Appl Sci Agric 9:165–169
Sirag N, Elhadi MM, Algaili AM, Hassan HM, Ohaj M (2014) Determination of total phenolic content and antioxidant activity of roselle (Hibiscus sabdariffa L.) calyx ethanolic extract. Stand Res J Pharm Pharmacol 1:34–39
Salmerón-Ruiz ML, Domínguez-Avila JA, Ayala-Zavala JF, Alvarez-Parrilla E, Villegas-Ochoa MA, Sáyago-Ayerdi SG, Valenzuela-Melendez M, González-Aguilar GA (2019) Optimization of total anthocyanin content and antioxidant activity of a Hibiscus sabdariffa infusion using response surface methodology. Biotecnia 21:114–122
Chikhoune A, Gagaoua M, Nanema KD, Souleymane AS, Hafid K, Aliane K, Hadjal S, Madani K, Sentandreu E, Sentandreu MÁ, Boudjellal A (2017) Antioxidant activity of Hibiscus sabdariffa extracts incorporated in an emulsion system containing whey proteins: oxidative stability and polyphenol--whey proteins interactions. Arab J Sci Eng 42:2247–2260
Purbowati ISM, Maksum A (2019) The antioxidant activity of Roselle (Hibiscus sabdariffa Linii) phenolic compounds in different variations microwave-assisted extraction time and power. IOP Conf Ser Earth Environ Sci 406:12005
Author information
Authors and Affiliations
Contributions
Tanvir Ahmed: formal data analysis, data calculation, and writing–original draft. Md Rahmatuzzaman Rana and Mohammad Afzal Hossain: methodology and writing–review and editing. Md Suzauddula: methodology, formal data analysis, data calculation, and writing–review and editing. Shakhawat Ullah: investigation, data curation, formal data analysis, data calculation, formal data analysis, and writing–review and editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmed, T., Rana, M.R., Hossain, M.A. et al. Optimization of ultrasound-assisted extraction using response surface methodology for total anthocyanin content, total phenolic content, and antioxidant activities of Roselle (Hibiscus sabdariffa L.) calyces and comparison with conventional Soxhlet extraction. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03881-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03881-y