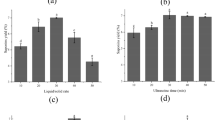
Abstract
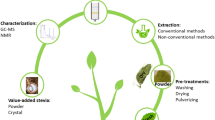
Nicotiana tabacum leaves are mainly used in the production of cigarettes and cigars all over the world, and a large amount of waste materials will be produced in the process. High-value scopoletin can be extracted and separated from waste materials, but there are some problems, such as low purity of products and inability of large-scale production. In this study, we established an efficient and stable method for producing high-purity scopoletin from waste tobacco by containing acetone reflux extracting with dynamic axial compression-high performance liquid chromatography (DAC-HPLC) separating. In particular, the extraction system makes the scopoletin fully extracted through continuous reflux, the DAC-HPLC system makes the high-purity scopoletin repeatedly obtained, and the DAC-HPLC system was first used for scopoletin. Finally, related to the waste tobacco power, the yield of scopoletin is 1.9%, and its HPLC purity is 99.94%. Additionally, a preparation method for the colorless needle crystal of scopoletin was established. Meanwhile, it has a strong antioxidant activity by testing the scavenging activity of the DPPH free radical and the Fe3+ reducing power. And its spatial structure was identified by an X-ray diffractometer, which laid a good foundation for in-depth study of scopoletin.
Graphical Abstract





Similar content being viewed by others
Availability of data and materials
All data generated or analyzed during this study are included in this manuscript and its supplementary information files.
Abbreviations
- DAC-HPLC:
-
Dynamic axial compression-high performance liquid chromatography
- C18:
-
Octadecylsilane (C18H40Si)
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- TA:
-
Tobacco
- SE:
-
Scopoletin extraction
- Ve:
-
Vitamin E
References
Andersen RA, Vaughn TH (1972) Short communication micro determination of scopoletin in Nicotiana tabacum. Phytochemistry 11:2593–2595
Lerat S, Babana AH, El OM, Daayf F, Beaudoin N, Bouarab K, Beaulieu C et al (2009) Streptomyces scabiei and its toxin thaxtomin A induce scopoletin biosynthesis in tobacco and Arabidopsis thaliana. Plant Cell Rep 28:1895–1903
Zhao HN, Cai K, Qin J et al (2017) Variance analysis of main metabolites in tobacco leaves before and after curing. Tob Sci Technol 50:61–67
Parama D, Girisa S, Khatoon E et al (2022) An overview of the pharmacological activities of scopoletin against different chronic diseases. Pharmacol Res 179:106202
Agati G, Brunetti C, Tuccio L et al (2022) Retrieving the in vivo scopoletin fluorescence excitation band allows the non-invasive investigation of the plant-pathogen early events in tobacco leaves. Front Microbiol 13:889878
Siwinska J, Siatkowska K, Olry A et al (2018) Scopoletin 8-hydroxylase: a novel enzyme involved in coumarin biosynthesis and iron-deficiency responses in Arabidopsis. J Exp Bot 69(7):1735–1748
Tsai HH, Rodríguez-Celma J, Lan P et al (2018) Scopoletin 8-hydroxylase-mediated fraxetin production is crucial for iron mobilization. Plant Physiol 177(1):194–207. https://doi.org/10.1104/pp.18.00178
Döll S, Kuhlmann M, Rutten T et al (2018) Accumulation of the coumarin scopolin under abiotic stress conditions is mediated by the Arabidopsis thaliana THO/TREX complex. Plant J 93(3):431–444
Sun H, Song N, Ma L et al (2017) Ethylene signalling is essential for the resistance of Nicotiana attenuata against Alternaria alternata and phytoalexin scopoletin biosynthesis. Plant Pathol 66(2):277–284
Choi RY, Ham JR, Lee HI et al (2017) Scopoletin supplementation ameliorates steatosis and inflammation in diabetic mice. Phytother Res 31(11):1795–1804
Zhao YX, Wang M, Dong WT et al (2019) Pharmacokinetics, bioavailability and metabolism of scopoletin in dog by ultra-high-performance liquid chromatography combined with linear ion trap-Orbitrap tandem mass spectrometry. Biomed Chromatogr 33(3):e4436
Fritig B, Hirth L, Ourisson G (1970) Biosynthesis of the Coumarins: Scopoletin formation in tobacco tissue cultures. Phytochemistry 9:1963–1975
Sun H, Wang L, Zhang B (2014) Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J Exp Bot 65:4305–4315
Fourcroy P, Sisó-Terraza P, Sudre D et al (2014) Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol 201(1):155–167
Stringlis IA, Yu K, Feussner K et al (2018) MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Natl Acad Sci USA 115(22):E5213–E5222
Hoengenaert L, Wouters M, Kim H et al (2022) Overexpression of the scopoletin biosynthetic pathway enhances lignocellulosic biomass processing. Sci adv 8(28):eabo738
Gao SN, Li S, Zhang LN et al (2012) Isolation and purification of scopoletin from Erycibe obtusifolia by high-speed counter-current chromatography. China Med Her 9(26):122–123
Sun JY, Zhou YQ, Li HY et al (2003) Determination of scopoletin in Cortex Mori from different sources. Chin Herb Med 03:77–79
Geng ZL, Zhang J, Yang MM et al (2013) Study on the chemical constituents of the tobacco leaves from Guizhou Province. J Yunnan Minzu University (Soc Sci) 22(2):79–81
Li L (2010) Study on the chemical composition of the fruit of Acanthopanax senticosus. Liaoning University of Traditional Chinese Medicine
Liu C, Hu Y, Li N et al (2009) Isolation of scopoletin from Physochlaine infundibularis Kuang by high-speed counter-current chromatography. Chem Res 20(02):101–103
Yang L, Wang MY, Zhang D et al (2006) Determination of scopoletin in Qinghao by HPLC. Chin J Exp Prescriptions 10:10–11
Sun YL, Liu WL, Liu YN et al (2018) Extraction technology of scopoletin from Rubus corchorifolius. Guizhou Agric Sci 46(05):120–122
Jokić S, Rajić M, Bilić B et al (2016) Supercritical extraction of scopoletin from Helichrysum italicum (Roth) G. Don flowers. Phytochem Anal 27(5):290–295
Chen X, Zhu P, Liu B et al (2018) Simultaneous determination of fourteen compounds of Hedyotis diffusa Willd extract in rats by UHPLC-MS/MS method: application to pharmacokinetics and tissue distribution study. J Pharm Biomed Anal 159:490–512
Sun HR, Yao H, Shi GF (2010) Study on chemical constituents from waste tobacco leavesII. Anhui Agric Sci 38:8394–8409
Kazuki T, Ayumi K, Takashi W et al (2019) Chemical constituents from the aerial parts of impatiens hypophylla Makino var. hypophylla. Biochem Syst Ecol 83:10–12
Donald GC, Robert VB (1962) New syntheses in the coumarin series. J Org Chem 27:3083–3085
Lang’at TC, Song TT, Hu J et al (2003) A simple synthesis of 7,4’-dihydroxy-6-methoxyisoflavone, glycitein, the third soybean isoflavone. J Nat Prod 66:149–151
Fang Z, He GL, He L (2007) Microwave-assisted synthesis of scopolamine. West China Pharm J 22:302–303
He BT, Liu ZH, Li BZ et al (2022Aug 2) Advances in biosynthesis of scopoletin. Microb Cell Fact 21(1):152
Rajniak J, Giehl R, Chang E et al (2018) Biosynthesis of redox-active metabolites in response to iron deficiency in plants. Nat Chem Biol 14(5):442–450
Kai K, Mizutani M, Kawamura N (2008) Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by A 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J 55(6):989–999
Bayoumi SA, Rowan MG, Blagbrough IS et al (2008) Biosynthesis of scopoletin and scopolin in cassava roots during post-harvest physiological deterioration: the E-Z-isomerisation stage. Phytochemistry 69(17):2928–2936
Yao R, Zhao Y, Liu T et al (2017) Identification and functional characterization of a P-coumaroyl CoA 2’-hydroxylase involved in the biosynthesis of coumarin skeleton from Peucedanum praeruptorum Dunn. Plant Mol Biol 95(1–2):199–213
Geng ZL, Zhang J, Yang MG et al (2013) Study on the chemical composition of tobacco leaves in Guizhou (I). J Yunnan Univ National (Nat Sci Ed) 22:79–81
Chen Y, Wu Y, Li S et al (2020) Large-scale isolation and antitumor mechanism evaluation of compounds from the traditional Chinese medicine Cordycepsmilitaris. Eur J Med Chem 212:113142
Gao H, Huang L, Ding F et al (2018) Simultaneous purification of dihydrotanshinone, tanshinone I, cryptotanshinone, and tanshinone IIA from Salvia miltiorrhiza and their anti-inflammatory activities investigation. Sci Rep 8(1):8460
Dang J, Cui Y, Pei J et al (2018) Efficient separation of four antibacterial diterpenes from the roots of Salvia prattii using non-aqueous hydrophilic solid-phase extraction followed by preparative high-performance liquid chromatography. Molecules 23(3):623
Cong J (2013) Separation and purification of superoxide dismutase (SOD) in wheat seedlings and its antioxidant activity. Sichuan Agricultural University
Wang YL (2018) Preparation of phosphatized Bletilla striata polysaccharide and its application in cosmetics. Shanghai University of Applied Sciences
Adfa M, Yoshimura T, Komura K et al (2010) Antitermite activities of coumarin derivatives and scopoletin from Protium javanicum Burm. f. J Chem Ecol 36:720–726
Beh HK, Ismail Z, Asmawi MZ et al (2010) 7-Hy-droxy-6-meth-oxy-2H-chromen-2-one. Acta Crystallogr Sect E Struct Rep Online 66:o2138
Banožić M, Banjari I, Jakovljević M et al (2019) Optimization of ultrasound-assisted extraction of some bioactive compounds from tobacco waste. Molecules 24(8):1611
Laszlo C, Kaminski K, Guan H et al (2022) Fractionation and extraction optimization of potentially valuable compounds and their profiling in six varieties of two Nicotiana species. Molecules 27(22):8105
Lemos ASO, Florêncio JR, Pinto NCC et al (2020) Antifungal activity of the natural coumarin scopoletin against planktonic cells and biofilms from a multidrug-resistant Candida tropicalis strain. Front Microbiol 11:1525
Siwinska J, Kadzinski L, Banasiuk R et al (2014) Dentification of QTLs affecting scopolin and scopoletin biosynthesis in Arabidopsis thaliana. BMC Plant Biol 14:280
Sun X, Zhou D, Kandavelu P et al (2015) Structural insights into substrate specificity of feruloyl-CoA 6’-hydroxylase from Arabidopsis thaliana. Sci Rep 5:10355
Li J and Wu J (2016) Scopolin, a glycoside form of the phytoalexin scopoletin, is likely involved in the resistance of Nicotiana attenuata against Alternaria alternata. J Plant Pathol 98(3)
Chen Y, Wu Y, Liu L et al (2019) Study of the whole genome, methylome and transcriptome of Cordycepsmlitaris. Sci Rep 9(1):898
Siwinska J, Siatkowska K, Olry A (2018) Scopoletin 8-hydroxylase: a novel enzyme involved in coumarin biosynthesis and iron-deficiency responses in Arabidopsis. J Exp Bot 69:1735–1748
Tsai HH, Rodríguez CJ, Lan P et al (2018) Scopoletin 8-hydroxylase-mediated fraxetin production is crucial for iron mobilization. Plant Physiol 177(1):194–207
Sun X, Zhou D, Kandavelu P (2015) Structural insights into substrate specificity of feruloyl-CoA 6’-hydroxylase from Arabidopsis thaliana. Sci Rep 5:10355
Hu S, Feng J, Wang M et al (2022) Nrf1 is an indispensable redox-determining factor for mitochondrial homeostasis by integrating multi-hierarchical regulatory networks. Redox Biol 57:102
Zhu YP, Zheng Z, Hu S et al (2019) Unification of opposites between two antioxidant transcription factors Nrf1 and Nrf2 in mediating distinct cellular responses to the endoplasmic reticulum stressor tunicamycin. Antioxidants 9(1):4
Ma XF, Zhang YY, Guo FY et al (2020) Molecular characterization of a voltage-gated calcium channel and its potential role in the acaricidal action of scopoletin against Tetranychus cinnabarinus. Pestic Biochem Physiol 168:104618
Wang Y, Zhang K, Li T et al (2021) Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics 11(1):164–180
Narasimhan KKS, Jayakumar D, Velusamy P et al (2019) Morinda citrifolia and its active principle scopoletin mitigate protein aggregation and neuronal apoptosis through augmenting the DJ-1/Nrf2/ARE signaling pathway. Oxid Med Cell Longev 2019:2761041
Rong N, Yang R, Ibrahim IAA et al (2022) Cardioprotective role of scopoletin on isoproterenol-induced myocardial infarction in rats. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-022-04123-z
Wang X, Wan M, Zhang L et al (2022) ALA_PDT promotes ferroptosis-like death of Mycobacterium abscessus and antibiotic sterilization via oxidative stress. Antioxidants 11(3):546
Huang X, Nisar MF, Wang M et al (2020) UV-responsive AKBA@ZnO nanoparticles potential for PMLE protection and therapy. Mater Sci Eng C Mater Biol Appl 107:110254
Acknowledgements
We would like to thank the China National Tobacco Corporation’s Guizhou Provincial Company for providing the tobacco raw materials, and Ling Hui (Guangzhou) Biotech Co. Ltd for promoting the wide application of scopoletin in this study. J.W. Bartsch, Marburg, Germany for useful discussions and editing.
Funding
This work were supported by the National Science and Technology Major Project, China (Grant Number: 2012ZX09401009), the Chongqing Municipal Education Commission, China (Grant Number: KYYJ202001), the Fundamental Research Funds for Central Universities, China (Grant Number: 2022CDJYGRH-005&2022CDJYGRH-016), and the Science and Technology Foundation, China (Grant Number: QKHZC [2017]2960, QKHZC [2018]2822).
Author information
Authors and Affiliations
Contributions
Y.-J. C. designed the study and wrote the manuscript. M. C., W.-H. Z., and S.-S. Z. performed experiments. X.-K. S., T.-J. Z., X.-W. S., J.-W.Z., and Y.-D. C. contributed to some experiments. G.-X. W., L. Z., J. C., and Z.-B. L. supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Chen, M., Zhang, W. et al. Large-scale isolation of scopoletin from Nicotiana tabacum. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03778-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03778-w