Abstract
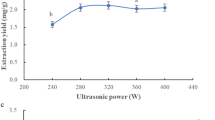
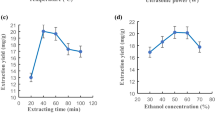
To research the optimization of ultrasonic-assisted extraction of total flavonoids from Cortex Lycii and its biological activities. Box-Behnken design experiment was used to optimize the extraction process, and AB-8 macroporous resin was used for purification. Its biological activities were studied by the inhibition rate of α-glucosidase and α- amylase and the scavenging rate of DPPH and ABTS. The optimal extraction parameters of total flavonoids from Cortex Lycii were as follows: the extraction temperature was 63 ℃, the ethanol volume fraction was 71%, the solid–liquid ratio was 28 mL/g, the extraction time was 61 min. Under this optimal condition, the yield of total flavonoids was 1.674 mg/g, which was consistent with the predicted value of 1.637 mg/g, and was 5.85 times higher than that of the traditional water extraction method. The inhibitory rate (IC50) of total flavonoids extract from Cortex Lycii on α-glucosidase was 3 μg/mL and on α-amylase was 5 μg/mL. The scavenging rate (IC50) of DPPH radical was 0.648 mg/mL and the scavenging rate (IC50) of ABTS radical IC50 was 0.012 mg/mL. The optimized extraction process with response surface design was reliable, which could be used for the extraction of total flavonoids from Cortex Lycii. It has strong hypoglycemic and antioxidant activities and has a good prospect for future utilization and development.





Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Zhang J, Guan S, Sun J, Liu T, Chen P et al (2015) Characterization and profiling of phenolic amides from Cortex Lycii by ultra-high performance liquid chromatography coupled with LTQ-Orbitrap mass spectrometry. Anal Bioanal Chem 407:581–595
Chin Y-W, Lim SW, Kim S-H, Shin D-Y, Suh Y-G et al (2003) Hepatoprotective pyrrole derivatives of Lycium chinense fruits. Bioorg Med Chem Lett 13:79–81
Gao D, Li Q, Liu Z, Li Y, Liu Z et al (2007) Hypoglycemic Effects and mechanisms of action of <i>Cortex Lycii Radicis</i> on alloxan-induced diabetic mice. Yakugaku Zasshi 127:1715–1721
Jung K, Chin Y-W, Kim YC, Kim J (2005) Potentially hepatoprotective glycolipid constituents of Lycium chinense fruits. Arch Pharmacal Res 28:1381–1385
Lee DG, Jung HJ, Woo E-R (2005) Antimicrobial property of (+)-lyoniresinol-3α-O-β-d-Glucopyranoside isolated from the root bark of Lycium chinense Miller against human pathogenic microorganisms. Arch Pharmacal Res 28:1031–1036
Ye Z, Huang Q, Ni HX, Wang D (2008) Cortex Lycii Radicis extracts improve insulin resistance and lipid metabolism in obese-diabetic rats. Phytother Res 22:1665–1670
Li YY, Di R, Baibado JT, Cheng YS, Huang YQ et al (2014) Identification of kukoamines as the novel markers for quality assessment of Lycii Cortex. Food Res Int 55:373–380
Qian D, Zhao Y, Yang G, Huang L (2017) Systematic review of chemical constituents in the genus Lycium (Solanaceae). Molecules 22:911
Yang Y, An Y, Wang W, Du N, Zhang J et al (2017) Nine compounds from the root bark of Lycium chinense and their anti-inflammatory activities. Acta Pharmaceutica Sinica B 7:491–495
Potterat O (2010) Goji (Lycium barbarum and L. chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med 76:7–19
Perez-Vizcaino F, Fraga CG (2018) Research trends in flavonoids and health. Arch Biochem Biophys 646:107–112
Chemat F, Rombaut N, Sicaire A-G, Meullemiestre A, Fabiano-Tixier A-S et al (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review Ultrasonics Sonochem 34:540–560
Chemat F, Vian MA, Fabiano-Tixier AS, Nutrizio M, Jambrak AR et al (2020) A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem 22:2325–2353
Li J, Wu C, Li F, Yu R, Wu X et al (2019) Optimization of ultrasound-assisted water extraction of flavonoids from Psidium guajava leaves by response surface analysis. Prep Biochem Biotechnol 49:21–29
Yao X, Zhu L, Chen Y, Tian J, Wang Y (2013) In vivo and in vitro antioxidant activity and alpha-glucosidase, alpha-amylase inhibitory effects of flavonoids from Cichorium glandulosum seeds. Food Chem 139:59–66
Striegel L, Kang B, Pilkenton SJ, Rychlik M, Apostolidis E (2015) Effect of black tea and black tea pomace polyphenols on alpha-glucosidase and alpha-amylase inhibition, relevant to type 2 diabetes prevention. Front Nutr 2:3
Eleazu CO, Eleazu KC, Iroaganachi M (2016) In vitro starch digestibility, α-amylase and α-glucosidase inhibitory capacities of raw and processed forms of three varieties of Livingstone potato ( Plectranthus esculentus ). Innov Food Sci Emerg Technol 37:37–43
Chen G, Fang C, Ran C, Tan Y, Yu Q et al (2019) Comparison of different extraction methods for polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int J Biol Macromol 130:903–914
Rozi P, Abuduwaili A, Mutailifu P, Gao Y, Rakhmanberdieva R et al (2019) Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int J Biol Macromol 131:97–106
Senrayan J, Venkatachalam S (2020) Ultrasonic acoustic-cavitation as a novel and emerging energy efficient technique for oil extraction from kapok seeds. Innov Food Sci Emerg Technol 62:102347
Pompeu DR, Silva EM, Rogez H (2009) Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using response surface methodology. Bioresour Technol 100:6076–6082
Jing CL, Dong XF, Tong JM (2015) Optimization of ultrasonic-assisted extraction of flavonoid compounds and antioxidants from alfalfa using response surface method. Molecules 20:15550–15571
Volpi N (2004) Application of high-performance capillary electrophoresis to the purification process of Escherichia coli K4 polysaccharide. J Chromatogr B Analyt Technol Biomed Life Sci 811:253–256
Zhou J, Zhang L, Li Q, Jin W, Chen W, et al. (2019) Simultaneous optimization for ultrasound-assisted extraction and antioxidant activity of flavonoids from Sophora flavescens using response surface methodology. Molecules 24:112
Yu M, Wang B, Qi Z, Xin G, Li W (2019) Response surface method was used to optimize the ultrasonic assisted extraction of flavonoids from Crinum asiaticum. Saudi J Biol Sci 26:2079–2084
Wu L, Li L, Chen S, Wang L, Lin X (2020) Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L leaves: Optimization, comparison and antioxidant activity. Separation and Purification Technol 247:117014
Zimare SB, Mankar GD, Barmukh RB (2021) Optimization of ultrasound-assisted extraction of total phenolics and flavonoids from the leaves of Lobelia nicotianifolia and their radical scavenging potential. Cur Res Green Sustain Chem 4:100109
Zheng B, Yuan Y, Xiang J, Jin W, Johnson JB et al (2022) Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: optimization, comparison and bioactivities. Lwt 154:112740
Tian J, Muhammad S, Chen A, Chen P, Wang J et al (2019) An experimental study exploring the influencing factors for ultrasonic-assisted extraction of flavonoid compounds from leaves of Amorpha fruticosa L. J For Res 30:1735–1741
Liyanapathirana C, Shahidi F (2005) Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem 93:47–56
Shahidi SA (2022) Effect of solvent type on ultrasound-assisted extraction of antioxidant compounds from Ficaria kochii: optimization by response surface methodology. Food Chem Toxicol 163:112981
Ademiluyi AO, Oboh G, Boligon AA, Athayde ML (2014) Effect of fermented soybean condiment supplemented diet on α-amylase and α-glucosidase activities in Streptozotocin-induced diabetic rats. Journal of Functional Foods 9:1–9
Lordan S, Smyth TJ, Soler-Vila A, Stanton C, Ross RP (2013) The alpha-amylase and alpha-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem 141:2170–2176
Mutailifu P, Nuerxiati R, Lu C, Huojiaaihemaiti H, Abuduwaili A et al (2022) Extraction, purification, and characterization of polysaccharides from Alhagi pseudoalhagi with antioxidant and hypoglycemic activities. Process Biochem 121:339–348
Deb PK, Khound P, Bhattacharjee S, Choudhury P, Sarma H et al (2021) Variation in chemical constituents, in-vitro bioactivity and toxicity profile among different parts of Clerodendrum glandulosum Lindl. (C. colebrookianum Walp.). S Afr J Bot 140:50–61
Jiang C, Chen Y, Ye X, Wang L, Shao J et al (2021) Three flavanols delay starch digestion by inhibiting alpha-amylase and binding with starch. Int J Biol Macromol 172:503–514
El Atki Y, Aouam I, El kamari F, Taroq A, Lyoussi B, et al (2019) Total phenolic and flavonoid contents and antioxidant activities of extracts from Teucrium polium growing wild in Morocco. Mater Today: Proc 13:777–783
Čopra-Janićijević A, Čulum D, Vidic D, Tahirović A, Klepo L et al (2018) Chemical composition and antioxidant activity of the endemic Crataegus microphylla Koch subsp. malyana K. I. Chr. & Janjić from Bosnia. Ind Crops Prod 113:75–79
Acknowledgements
This study was supported by the National Key Clinical Specialty Construction Project (Clinical Pharmacy) and the High-Level Clinical Key Specialty (Clinical Pharmacy) in Guangdong Province.
Funding
This research work was financially supported by the Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515110565), the Medical Scientific Research Foundation of Guangdong Province of China (No. B2021051), the Scientific Research Project of Traditional Chinese Medicine Bureau of Guangdong Province of China (No. 20212119), the Science and Technology Program of Guangzhou, China (No. 202201010530), and the College Students’ innovation and entrepreneurship training program (No. 202210573026).
Author information
Authors and Affiliations
Contributions
Methodology, YW and XZ; investigation, YW, XZ, and HT; formal analysis, YW, XZ, and HT; resources, YW; writing—original draft preparation, YW; writing—review and editing, Y W and XZ; supervision, YW and BL; project administration, YW and BL; funding acquisition, YW.
Corresponding authors
Ethics declarations
Ethics approval
This article contains no studies with human participants or animals performed by any of the authors.
Consent to participate
Not applicable. This study did not use any human or animal subjects.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, Y., Zeng, X., Tan, H. et al. Optimization of extraction process of total flavonoids from Cortex Lycii and its biological activities. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03768-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03768-y