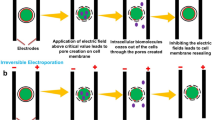
Abstract
Among many existing microalgae, Haematococcus pluvialis has been known as the potential source of natural astaxanthin which have remarkable antioxidant properties and wide applications in pharmaceutical, aquaculture, therapeutic, and nutraceutical sectors. However, the high cost of extracting this pigment makes the cost of astaxanthin very high. Latest research has shown that pulsed electric field (PEF) is simple yet sensitive technique to permeabilize the thick cell wall of H. pluvialis and extract the pigment. Although there exists plenty of conventional techniques for large scale extraction of value-added compounds from microalgae, none of them can be applied successfully. In PEF controlling and optimizing the parameters makes this technique facile to get the desired products accounting to little loss in cell viability. The review provides a close view on the astaxanthin accumulation under stress conditions in microalgae. It also documents biocompatible electric treatment for cell permeabilization, the capacity of microalgae to survive and regenerate the pool of extracted compounds. This communication attempts to review the challenges and perspectives of research in this field of work.
Graphical abstract



Similar content being viewed by others
References
Pan-utai W, Boonpok S, Pornpukdeewattana S (2021) Combination of mechanical and chemical extraction of astaxanthin from Haematococcus pluvialis and its properties of microencapsulation. Biocatal. Agric. Biotechnol 33:101979
Oslan SNH, Shoparwe NF, Yusoff AH, Rahim AA, Chang CS, Tan JS, Oslan SN, Arumugam K, Ariff AB, Sulaiman AZ (2021) A review on Haematococcus pluvialis bioprocess optimization of green and red stage culture conditions for the production of natural astaxanthin. Biomol 11(2):256
Sirotiya, V, Ahirwar A, Mourya M, Khan MJ, Rai, A, Kwatra R, Sharma AK, Harish, Schoefs B, Marchand J, Varjani S, Vinayak V (2022). Astaxanthin bioaccumulation in microalgae under environmental stress simulated in industrial effluents highlighting prospects of Haematococcus pluvialis: knowledge gaps and prospective approaches, Phytochem Rev (In press).
Ahirwar A, Meignen G, Khan MJ, Sirotiya V, Scarsini M, Roux S, Marchand J, Schoefs B, Vinayak V (2021) Light modulates transcriptomic dynamics upregulating astaxanthin accumulation in Haematococcus: a review. Bioresour. Technol 340:125707
Nagarajan D, Varjani S, Lee D-J, Chang J-S (2021) Sustainable aquaculture and animal feed from microalgae–nutritive value and techno-functional components. Renew. Sust. Energ. Rev150:111549
Matos J, Cardoso C, Bandarra N, Afonso C (2017) Microalgae as healthy ingredients for functional food: a review. Food Funct 8(8):2672–2685
Luo Q, Bian C, Tao M, Huang Y, Zheng Y, Lv Y, Li J, Wang C, You X, Jia B (2019) Genome and transcriptome sequencing of the astaxanthin-producing green microalga. Haematococcus pluvialis Genome Biol and Evol (GBE) 11(1):166–173
Ambati RR, Phang S-M, Ravi S, Aswathanarayana RG (2014) Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs 12(1):128–152
Yuan JP, Peng J, Yin K, Wang JH (2011) Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res 55(1):150–165
Chou H-Y, Ma D-L, Leung C-H, Chiu C-C, Hour T-C, Wang H-MD (2020) Purified astaxanthin from Haematococcus pluvialis promotes tissue regeneration by reducing oxidative stress and the secretion of collagen in vitro and in vivo. Oxid Med Cell Longev: 4946902
Esatbeyoglu T, Rimbach G (2017) Canthaxanthin: from molecule to function. Mol Nutr Food Res 61(6):1600469
Hong ME, Choi HI, Kwak HS, Hwang S-W, Sung YJ, Chang WS, Sim SJ (2018) Rapid selection of astaxanthin-hyperproducing Haematococcus mutant via azide-based colorimetric assay combined with oil-based astaxanthin extraction. Bioresor Techno 267:175–181
Ahirwar A, Meignen G, Khan M, Khan N, Rai A, Schoefs B, Marchand J, Varjani S, Vinayak V (2021) Nanotechnological approaches to disrupt the rigid cell walled microalgae grown in wastewater for value-added biocompounds: commercial applications, challenges, and breakthrough. Biomass Convers Biorefin 1–26
Ren Y, Deng J, Huang J, Wu Z, Yi L, Bi Y, Chen F (2021) Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: advances and outlook. Bioresour Techno 340:125736
Samorì C, Pezzolesi L, Galletti P, Semeraro M, Tagliavini E (2019) Extraction and milking of astaxanthin from Haematococcus pluvialis cultures. Green Chem 21(13):3621–3628
V Vinayak KM Manoylov H Gateau V Blanckaert J Hérault Pencreac’h G, Marchand J, Gordon R, Schoefs B 2015 Diatom milking: a review and new approaches Mar Drugs 13 5 2629 2665
Bauer A, Minceva M (2021) Techno-economic analysis of a new downstream process for the production of astaxanthin from the microalgae Haematococcus pluvialis. Bioresour Bioprocess 8(1):1–18
Khoo KS, Lee SY, Ooi CW, Fu X, Miao X, Ling TC, Show PL (2019) Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour Techno 288:121606
Catchpole O, Moreno T, Montañes F, Tallon S (2018) Perspectives on processing of high value lipids using supercritical fluids. J Supercrit Fluids 134:260–268
Bauer A, Minceva M (2019) Direct extraction of astaxanthin from the microalgae Haematococcus pluvialis using liquid–liquid chromatography. RSC Adv 9(40):22779–22789
Ye Z, Tan XH, Liu ZW, Aadil RM, Tan YC, Inam-ur-Raheem M (2020) Mechanisms of breakdown of Haematococcus pluvialis cell wall by ionic liquids, hydrochloric acid and multi-enzyme treatment. Int J Food Sci 55(9):3182–3189
Liu ZW, Yue Z, Zeng XA, Cheng JH, Aadil RM (2019) Ionic liquid as an effective solvent for cell wall deconstructing through astaxanthin extraction from Haematococcus pluvialis. Int J Food Sci 54(2):583–590
Khoo KS, Chew KW, Yew GY, Manickam S, Ooi CW, Show PL (2020) Integrated ultrasound-assisted liquid biphasic flotation for efficient extraction of astaxanthin from Haematococcus pluvialis. Ultrason Sonochem 67:105052
Saini RK, Keum Y-S (2018) Carotenoid extraction methods: a review of recent developments. Food chem 240:90–103
Choi S-A, Oh Y-K, Lee J, Sim SJ, Hong ME, Park J-Y, Kim M-S, Kim SW, Lee J-S (2019) High-efficiency cell disruption and astaxanthin recovery from Haematococcus pluvialis cyst cells using room-temperature imidazolium-based ionic liquid/water mixtures. Bioresour Techno 274:120–126
Haque F, Dutta A, Thimmanagari M, Chiang YW (2016) Intensified green production of astaxanthin from Haematococcus pluvialis. Food Bioprod Process 99:1–11
Liu Y-H, Alimujiang A, Wang X, Luo S-W, Balamurugan S, Yang W-D, Liu J-S, Zhang L, Li H-Y (2019) Ethanol induced jasmonate pathway promotes astaxanthin hyperaccumulation in Haematococcus pluvialis. Bioresour. Technol 289:121720
Irshad M, Myint AA, Hong ME, Kim J, Sim SJ (2019) One-pot, simultaneous cell wall disruption and complete extraction of astaxanthin from Haematococcus pluvialis at room temperature. ACS Sustain Chem Eng 7(16):13898–13910
Butler T, Golan Y (2020) Astaxanthin production from microalgae. Microalgae Biotechno Food, Health and High Value Produc. Springer, pp 175–242
Lee SY, Cho JM, Chang YK, Oh Y-K (2017) Cell disruption and lipid extraction for microalgal biorefineries: a review. Bioresour Techno 244:1317–1328
Martínez JM, Delso C, Álvarez I, Raso J (2020) Pulsed electric field-assisted extraction of valuable compounds from microorganisms. Compr Rev Food Sci Food Saf 19(2):530–552
Gateau H, Blanckaert V, Veidl B, Burlet-Schiltz O, Pichereaux C, Gargaros A, Marchand J, Schoefs B (2021) Application of pulsed electric fields for the biocompatible extraction of proteins from the microalga Haematococcus pluvialis. Bioelectrochem 137:107588
Haberkorn I, Buchmann L, Häusermann I, Mathys A (2021) Nanosecond pulsed electric field processing of microalgae based biorefineries governs growth promotion or selective inactivation based on underlying microbial ecosystems. Bioresour Techno 319:124173
Liu C, Hu B, Cheng Y, Guo Y, Yao W, Qian H (2021) Carotenoids from fungi and microalgae: a review on their recent production, extraction, and developments. Bioresour. Techno 337:125398
Nitsos C, Filali R, Taidi B, Lemaire J (2020) Current and novel approaches to downstream processing of microalgae: a review. Biotechnol. Adv 45:107650
Rokicka M, Zieliński M, Dudek M, Dębowski M (2021) Effects of ultrasonic and microwave pretreatment on lipid extraction of microalgae and methane production from the residual extracted biomass. Bioenergy Res 14(3):752–760
Khoo KS, Ooi CW, Chew KW, Foo SC, Lim JW, Tao Y, Jiang N, Ho S-H, Show PL (2021) Permeabilization of Haematococcus pluvialis and solid-liquid extraction of astaxanthin by CO2-based alkyl carbamate ionic liquids. Chem. Eng. J 411:128510
Khan MJ, Mangesh H, Ahirwar A, Schoefs B, Pugazhendhi A, Varjani S, Rajendran K, Bhatia SK, Saratale GD, Saratale RG (2021) Insights into diatom microalgal farming for treatment of wastewater and pretreatment of algal cells by ultrasonication for value creation. Environ Res 201:111550
Vinayak V (2022) Algae as sustainable food in space missions. Biomass, Biofuels, Biochem Elsevier, pp 517–540
Nguyen TT, Thanh BX, Ngo HH, Nguyen TTD, Nguyen KQ, Nguyen HH, Huynh KPH, Némery J, Fujioka T, Duong CH, Dang BT, Varjani S (2021). Nutrient recovery and microalgae biomass production from urine by membrane photobioreactor at low biomass retention times. Sci Total Environ 785: 147423
Nguyen TTD, Bui XT, Nguyen TT, Ngo HH, Lin KYA, Lin C, Le LT, Dang BT, Bui MH, Varjani S (2022). Co-culture of microalgae-activated sludge in sequencing batch photobioreactor systems: Effects of natural and artificial lighting on wastewater treatment. Bioresour Techno 343:126091
Hu Q, Huang D, Li A, Hu Z, Gao Z, Yang Y, Wang C (2021) Transcriptome-based analysis of the effects of salicylic acid and high light on lipid and astaxanthin accumulation in Haematococcus pluvialis. Biotechnol Biofuels 14(1):1–20
Fitriana HN, Lee SY, Choi S-A, Lee J, Kim B, Lee J-S, Oh Y-K (2021) Electric Stimulation of Astaxanthin Biosynthesis in Haematococcus pluvialis. Appl Sci 11(8):3348
Devadas VV, Khoo KS, Chia WY, Chew KW, Munawaroh HSH, Lam M-K, Lim J-W, Ho Y-C, Lee KT, Show PL (2021) Algae biopolymer towards sustainable circular economy. Bioresour. Technol 325:124702
Praveenkumar R, Lee K, Lee J, Oh Y-K (2015) Breaking dormancy: an energy-efficient means of recovering astaxanthin from microalgae. Green Chem 17(2):1226–1234
Rawat I, Kumar RR, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88(10):3411–3424
Razon LF, Tan RR (2011) Net energy analysis of the production of biodiesel and biogas from the microalgae: Haematococcus pluvialis and Nannochloropsis. Appl Energy 88(10):3507–3514
Jaeschke DP, Menegol T, Rech R, Mercali GD, Marczak LDF (2016) Carotenoid and lipid extraction from Heterochlorella luteoviridis using moderate electric field and ethanol. Process Biochem 51(10):1636–1643
Grimi N, Dubois A, Marchal L, Jubeau S, Lebovka N, Vorobiev E (2014) Selective extraction from microalgae Nannochloropsis sp. using different methods of cell disruption. Bioresour Technol 153:254–259
Zhang R, Gu X, Xu G, Fu X (2021) Improving the lipid extraction yield from Chlorella based on the controllable electroporation of cell membrane by pulsed electric field. Bioresour Technol 330:124933
Kempkes MA (2016) Pulsed electric fields for algal extraction and predator control. Handb Electroporat:1–16
Martínez JM, Gojkovic Z, Ferro L, Maza M, Álvarez I, Raso J, Funk C (2019) Use of pulsed electric field permeabilization to extract astaxanthin from the Nordic microalga Haematococcus pluvialis. Bioresour Technol 289:121694
Ruen-ngam D, Shotipruk A, Pavasant P (2010) Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep Sci Technol 46(1):64–70
Fan Y, Niu Z, Xu C, Yang L, Chen F, Zhang H (2019) Biocompatible protic ionic liquids-based microwave-assisted liquid-solid extraction of astaxanthin from Haematococcus pluvialis. Ind Crops Prod 141:111809
Kumar PS, Varjani SJ, Suganya S (2018) Treatment of dye wastewater using an ultrasonic aided nanoparticle stacked activated carbon: kinetic and isotherm modelling. Bioresour Techno 250:716–722
Reyes FA, Mendiola JA, Ibanez E, del Valle JM (2014) Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J Supercrit Fluids 92:75–83
Cheng X, Qi Z, Burdyny T, Kong T, Sinton D (2018) Low pressure supercritical CO2 extraction of astaxanthin from Haematococcus pluvialis demonstrated on a microfluidic chip. Bioresour Technol 250:481–485
Show K-Y, Lee D-J, Tay J-H, Lee T-M, Chang J-S (2015) Microalgal drying and cell disruption–recent advances. Bioresour Technol 184:258–266
Desai RK, Streefland M, Wijffels RH, Eppink MH (2016) Novel astaxanthin extraction from Haematococcus pluvialis using cell permeabilising ionic liquids. Green Chem 18(5):1261–1267
Salatti-Dorado JA, García-Gómez D, Rodriguez-Ruiz V, Gueguen V, Pavon-Djavid G, Rubio S (2019) Multifunctional green supramolecular solvents for cost-effective production of highly stable astaxanthin-rich formulations from Haematococcus pluvialis. Food Chem 279:294–302
Puértolas E, Barba FJ (2016) Electrotechnologies applied to valorization of by-products from food industry: main findings, energy and economic cost of their industrialization. Food Bioprod Process 100:172–184
Jacobsen C, García-Moreno PJ, Mendes AC, Mateiu RV, Chronakis IS (2018) Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Annu Rev Food Sci Technol 9:525–549
bin Azmi AA, Sankaran R, Show PL, Ling TC, Tao Y, Munawaroh HSH, San Kong P, Lee D-J, Chang J-S (2020) Current application of electrical pre-treatment for enhanced microalgal biomolecules extraction. Bioresour Technol. 302:122874
Zhao W, Tang Y, Lu L, Chen X, Li C (2014) Pulsed electric fields processing of protein-based foods. Food Bioproc Tech 7(1):114–125
Kotnik T, Frey W, Sack M, Meglič SH, Peterka M, Miklavčič D (2015) Electroporation-based applications in biotechnology. Trends Biotechnol 33(8):480–488
Boussetta N, Lesaint O, Vorobiev E (2013) A study of mechanisms involved during the extraction of polyphenols from grape seeds by pulsed electrical discharges. Innov Food Sci Emerg Technol 19:124–132
Monfort S, Saldaña G, Condón S, Raso J, Álvarez I (2012) Inactivation of Salmonella spp. in liquid whole egg using pulsed electric fields, heat, and additives. Food Microbiol. 30 (2):393–399
Zimmermann U, Vienken J (1982) Electric field-induced cell-to-cell fusion. J Membr Biol 67(1):165–182
Mourya M, Khan MJ, Ahirwar A, Schoefs B, Marchand J, Rai A, Varjani S, Rajendran K, Banu JR, Vinayak V (2021) Latest trends and developments in microalgae as potential source for biofuels: The case of diatoms. Fuel:122738
Fauster T, Giancaterino M, Pittia P, Jaeger H (2020) Effect of pulsed electric field pretreatment on shrinkage, rehydration capacity and texture of freeze-dried plant materials. LWT 121:108937
Ben-Or A, Rubinsky B (2010) Experimental studies on irreversible electroporation of cells. Irreversible Electroporation. Springer, pp 63–83
Varjani S, Rakholiya P, Shindhal T, Shah AV, Ngo HH, (2021). Trends in dye industry effluent treatment and recovery of value added products. J. Water Process. Eng 39: 101734
Luengo E, Martínez JM, Bordetas A, Álvarez I, Raso J (2015) Influence of the treatment medium temperature on lutein extraction assisted by pulsed electric fields from Chlorella vulgaris. Innov Food Sci Emerg Technol 29:15–22
Shindhal T, Rakholiya P, Varjani S, Pandey A, Ngo HH, Guo W, Ng HY, Taherzadeh MJ (2020) A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 12(1):70–87
Zheng H, Wang Y, Li S, Nagarajan D, Varjani S, Lee D-J, Chang J-S (2022) Recent advances in lutein production from microalgae. Renew Sust Energ Rev. 153:111795
Adesra A, Srivastava VK, Varjani S (2021) Valorization of dairy wastes: integrative approaches for value added products. Indian J. Microbiol:1–9
Yadav G, Mishra A, Ghosh P, Sindhu R, Vinayak V, Pugazhendhi A (2021) Technical, economic and environmental feasibility of resource recovery technologies from wastewater. Sci Total Environ 796:149022
Khan MJ, Bawra N, Verma A, Kumar V, Pugazhendhi A, Joshi KB, Vinayak V (2021) Cultivation of diatom Pinnularia saprophila for lipid production: a comparison of methods for harvesting the lipid from the cells. Bioresour Techno 319:124129
Khan MJ, Das S, Vinayak V, Pant D, Ganghrekar M (2021) Live diatoms as potential biocatalyst in a microbial fuel cell for harvesting continuous diafuel, carotenoids and bioelectricity. Chemosphere 291:132841
Vinayak V, Khan MJ, Varjani S, Saratale GD, Saratale RG, Bhatia SK (2021) Microbial fuel cells for remediation of environmental pollutants and value addition: special focus on coupling diatom microbial fuel cells with photocatalytic and photoelectric fuel cells. J Biotechnol 338:5–19
Khan MJ, Suryavanshi VJ, Joshi KB, Gangadharan P, Vinayak V (2022) Photosynthetic microalgal microbial fuel cells and its future upscaling aspects. Handbook of Alg Biofu Elsevier 363–384
Leong YK, Chen C-Y, Varjani S, Chang J-S (2021) Producing fucoxanthin from algae–Recent advances in cultivation strategies and downstream processing. Bioresour Technol:126170
Seth K, Kumar A, Rastogi RP, Meena M, Vinayak V (2021) Bioprospecting of fucoxanthin from diatoms—challenges and perspectives. Algal Res 60:102475
Kumar V, Kashyap M, Gautam S, Shukla P, Joshi KB, Vinayak V (2018) Fast Fourier infrared spectroscopy to characterize the biochemical composition in diatoms. J Bio Sci 43(4):717–729
Varjani S, Shah AV, Vyas S, Srivastava VK (2021) Processes and prospects on valorizing solid waste for the production of valuable products employing bio-routes: a systematic review. Chemosphere 282:130954
Khan MJ, Singh N, Mishra S, Ahirwar A, Bast F, Schoefs B, Marchand J, Rajendran K, Banu JR, Saratale GD (2021) Impact of light on microalgal photosynthetic microbial fuel cells and removal of pollutants by nanoadsorbent biopolymers: updates, challenges and innovations. Chemosphere 288:132589
Shah AV, Srivastava VK, Mohanty SS, Varjani S (2021) Municipal solid waste as a sustainable resource for energy production: state-of-the-art review. J. Environ. Chem. Eng.:105717
Khan MJ, Rai A, Ahirwar A, Sirotiya V, Mourya M, Mishra S, Schoefs B, Marchand J, Bhatia SK, Varjani S (2021) Diatom microalgae as smart nanocontainers for biosensing wastewater pollutants: recent trends and innovations. Bioengineered 12(2):9531–9549
Chen CY, Kuo EW, Nagarajan D, Dong CD, Lee DJ, Varjani S, Lam SS, Chang JS (2021). Semi-batch cultivation of Chlorella sorokiniana AK-1 with dual carriers for the effective treatment of full strength piggery wastewater treatment. Bioresour Techno 326: 124773
Dang BT, Bui XT, Tran, HDPH, Ngo H, Nghiem LD, Hoang TKD, Nguyen PT, Nguyen HH, Quyen Vo T, Lin C, Lin KYA, Varjani S (2022). Current application of algae derivatives for bioplastic production: a review. Bioresour. Technol. 347: 126698
Colusse GA, Duarte MER, de Carvalho JC, Noseda MD (2021) Production of astaxanthin by Haematococcus pluvialis: lab processes to scale up including the cost considerations. Glob Persp on Astaxan:121–130
Ota S, Morita A, Ohnuki S, Hirata A, Sekida S, Okuda K, Ohya Y, Kawano S (2018) Carotenoid dynamics and lipid droplet containing astaxanthin in response to light in the green alga Haematococcus pluvialis. Sci Rep 8(1):1–10
Liu ZW, Zeng XA, Cheng JH, Liu DB, Aadil RM (2018) The efficiency and comparison of novel techniques for cell wall disruption in astaxanthin extraction from Haematococcus pluvialis. Int J Food Sci 53(9):2212–2219
Bustos-Garza C, Yáñez-Fernández J, Barragán-Huerta BE (2013) Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Int Food Res 54(1):641–649
Poojary MM, Barba FJ, Aliakbarian B, Donsì F, Pataro G, Dias DA, Juliano P (2016) Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar Drugs 14(11):214
Silve A, Kian CB, Papachristou I, Kubisch C, Nazarova N, Wüstner R, Leber K, Straessner R, Frey W (2018) Incubation time after pulsed electric field treatment of microalgae enhances the efficiency of extraction processes and enables the reduction of specific treatment energy. Bioresour Techno 269:179–187
Scherer D, Krust D, Frey W, Mueller G, Nick P, Gusbeth C (2019) Pulsed electric field (PEF)-assisted protein recovery from Chlorella vulgaris is mediated by an enzymatic process after cell death. Algal Res 41:101536
Chittapun S, Jonjaroen V, Khumrangsee K, Charoenrat T (2020) C-phycocyanin extraction from two freshwater cyanobacteria by freeze thaw and pulsed electric field techniques to improve extraction efficiency and purity. Algal Res 46:101789
Tosuner ZV, Urek RO (2021) Effects of nutrients on lipids and biodiesel production potential of Haematococcus pluvialis. Environ Eng Manag 20 (8)
Marinho YF, Malafaia CB, de Araujo KS, da Silva TD, dos Santos APF, de Moraes LB, Galvez AO (2021) Evaluation of the influence of different culture media on growth, life cycle, biochemical composition, and astaxanthin production in Haematococcus pluvialis. Aquac Int 29(2):757–778
Butler TO, Guimarães B (2021) Industrial perspective on downstream processing of Haematococcus pluvialis. Global Perspectives on Astaxanthin. Elsevier, pp 283–311
Coustets M, Joubert-Durigneux V, Hérault J, Schoefs B, Blanckaert V, Garnier J-P, Teissié J (2015) Optimization of protein electroextraction from microalgae by a flow process. Bioelectrochem 103:74–81
Esser AT, Smith KC, Gowrishankar T, Vasilkoski Z, Weaver JC (2010) Mechanisms for the intracellular manipulation of organelles by conventional electroporation. Biophys J 98(11):2506–2514
Pakhomov AG, Gianulis E, Vernier PT, Semenov I, Xiao S (1848) Pakhomova ON (2015) Multiple nanosecond electric pulses increase the number but not the size of long-lived nanopores in the cell membrane. Biochim Biophys Acta Biomembr 4:958–966
Galarza JI, Vega BOA, Villón J, Henríquez V (2019) Deesterification of astaxanthin and intermediate esters from Haematococcus pluvialis subjected to stress. Biotechnol. Rep. 23:e00351
Kim JH, Affan A, Jang J, Kang M-H, Ko A-R, Jeon S-M, Oh C, Heo S-J, Lee Y-H, Ju S-J (2015) Morphological, molecular, and biochemical characterization of astaxanthin-producing green microalga Haematococcus sp. KORDI03 (Haematococcaceae, Chlorophyta) isolated from Korea. J. Microbiol. Biotechnol 25 (2):238–246
Khan MJ, Gordon R, Varjani S, Vinayak V (2022). Employing newly developed plastic bubble wrap technique for biofuel production from diatoms cultivated in discarded plastic waste, Sci Total Environ 153667 (In press).
Eing C, Goettel M, Straessner R, Gusbeth C, Frey W (2013) Pulsed electric field treatment of microalgae—benefits for microalgae biomass processing. IEEE Transac on Plasma Sci 41(10):2901–2907
Alhattab M, Kermanshahi-Pour A, Brooks MS-L (2019) Microalgae disruption techniques for product recovery: influence of cell wall composition. J Appl Psychol 31(1):61–88
Flisar K, Meglic SH, Morelj J, Golob J, Miklavcic D (2014) Testing a prototype pulse generator for a continuous flow system and its use for E. coli inactivation and microalgae lipid extraction. Bioelectrochem 100:44–51
Luengo E, Condón-Abanto S, Álvarez I, Raso J (2014) Effect of pulsed electric field treatments on permeabilization and extraction of pigments from Chlorella vulgaris. J Membr Biol 247(12):1269–1277
Guionet A, Hosseini B, Teissié J, Akiyama H, Hosseini H (2017) A new mechanism for efficient hydrocarbon electro-extraction from Botryococcus braunii. Biotechnol Biofuels 10(1):1–9
Recht L, Zarka A, Boussiba S (2012) Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl Microbiol Biotechno 94(6):1495–1503
Mularczyk M, Michalak I, Marycz K (2020) Astaxanthin and other nutrients from Haematococcus pluvialis—multifunctional applications. Mar Drugs 18(9):459
Postma P, Pataro G, Capitoli M, Barbosa M, Wijffels RH, Eppink M, Olivieri G, Ferrari G (2016) Selective extraction of intracellular components from the microalga Chlorella vulgaris by combined pulsed electric field–temperature treatment. Bioresour Technol 203:80–88
Coustets M, Al-Karablieh N, Thomsen C, Teissié J (2013) Flow process for electroextraction of total proteins from microalgae. J Memb Biol 246(10):751–760
Parniakov O, Barba FJ, Grimi N, Marchal L, Jubeau S, Lebovka N, Vorobiev E (2015) Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae Nannochloropsis. Algal Res 8:128–134
Goettel M, Eing C, Gusbeth C, Straessner R, Frey W (2013) Pulsed electric field assisted extraction of intracellular valuables from microalgae. Algal Res 2(4):401–408
Chen Z, Lee WG (2019) Electroporation for microalgal biofuels: a review. Sustain Energ Fuels 3(11):2954–2967
Foltz G (2012) Algae lysis with pulsed electric fields. California Polytechnic State University, San Luis Obispo. Master Thesis
Frey W, Gusbeth C, Schwartz T (2013) Inactivation of Pseudomonas putida by pulsed electric field treatment: a study on the correlation of treatment parameters and inactivation efficiency in the short-pulse range. J Membr Biol 246(10):769–781
Garoma T, Janda D (2016) Investigation of the effects of microalgal cell concentration and electroporation, microwave and ultrasonication on lipid extraction efficiency. Renew Energy 86:117–123
Fritsch C, Staebler A, Happel A, Cubero Márquez MA, Aguiló-Aguayo I, Abadias M, Gallur M, Cigognini IM, Montanari A, López MJ (2017) Processing, valorization and application of bio-waste derived compounds from potato, tomato, olive and cereals: a review. Sustainabi 9(8):1492
Guo B, Yang B, Silve A, Akaberi S, Scherer D, Papachristou I, Frey W, Hornung U, Dahmen N (2019) Hydrothermal liquefaction of residual microalgae biomass after pulsed electric field-assisted valuables extraction. Algal Res. 43:101650
Fasolin LH, Rodrigues RM, Pereira RN (2021) Effects of electric fields and electromagnetic wave on food structure and functionality. Food Struct Functionality Elsevier, pp 95–113
Liu C, Grimi N, Bals O, Lebovka N, Vorobiev E (2021) Effects of pulsed electric fields and preliminary vacuum drying on freezing assisted processes in potato tissue. Food Bioprod Process 125:126–133
Parniakov O, Barba FJ, Grimi N, Lebovka N, Vorobiev E (2016) Extraction assisted by pulsed electric energy as a potential tool for green and sustainable recovery of nutritionally valuable compounds from mango peels. Food Chem 192:842–848
Sánchez-Vega R, Garde-Cerdán T, Rodríguez-Roque MJ, Elez-Martínez P, Martín-Belloso O (2020) High-intensity pulsed electric fields or thermal treatment of broccoli juice: the effects of processing on minerals and free amino acids. Eur Food Res Techno 246(3):539–548
Picart-Palmade L, Cunault C, Chevalier-Lucia D, Belleville M-P, Marchesseau S (2019) Potentialities and limits of some non-thermal technologies to improve sustainability of food processing. Front Nutr 5:130
Misra R, Guldhe A, Singh P, Rawat I, Bux F (2014) Electrochemical harvesting process for microalgae by using nonsacrificial carbon electrode: a sustainable approach for biodiesel production. Chem Eng 255:327–333
Sun H, Guan B, Kong Q, Geng Z, Wang N (2016) Repeated cultivation: non-cell disruption extraction of astaxanthin for Haematococcus pluvialis. Sci Rep 6(1):1–12
Mishra B, Varjani S, Agarwal DC, Mandal SK, Ngo HH, Taherzadeh MJ, Chang JS, You S, Guo W (2020). Engineering biocatalytic material for the remediation of pollutants: a comprehensive review. Environ Techno Innov20: 101063
Luengo E, Martínez JM, Coustets M, Álvarez I, Teissié J, Rols M-P, Raso J (2015) A comparative study on the effects of millisecond-and microsecond-pulsed electric field treatments on the permeabilization and extraction of pigments from Chlorella vulgaris. J Membr Biol 248(5):883–891
‘t Lam GP, van der Kolk JA, Chordia A, Vermuë MH, Olivieri G, Eppink MH, Wijffels RH 2017 Mild and selective protein release of cell wall deficient microalgae with pulsed electric field ACS Sustain ChemEng 7 6046 6053
Liu X, Zhang M, Liu H, Zhou A, Cao Y, Liu X (2018) Preliminary characterization of the structure and immunostimulatory and anti-aging properties of the polysaccharide fraction of Haematococcus pluvialis. RSC Adv 17:9243–9252
Lai YS, Parameswaran P, Li A, Baez M, Rittmann BE (2014) Effects of pulsed electric field treatment on enhancing lipid recovery from the microalga, Scenedesmus. Bioresour Techno 173:457–461
Machado FR Jr, Trevisol TC, Boschetto DL, Burkert JF, Ferreira SR, Oliveira JV, Burkert CAV (2016) Technological process for cell disruption, extraction and encapsulation of astaxanthin from Haematococcus pluvialis. J Biotechnol 218:108–114
Praveenkumar R, Lee J, Vijayan D, Lee SY, Lee K, Sim SJ, Hong ME, Kim Y-E, Oh Y-K (2020) Morphological change and cell disruption of Haematococcus pluvialis cyst during high-pressure homogenization for astaxanthin recovery. Appl Sci 10(2):513
Suktham K, Daisuk P, Shotipruk A (2021) Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia L.(Rubiaceae): Errata and review of technological development and prospects. Sep Purif Technol 256:117844
Al Jitan S, Alkhoori SA, Yousef LF (2018) Phenolic acids from plants: extraction and application to human health. Stud Nat Prod Chem 58:389–417
Molino A, Mehariya S, Iovine A, Larocca V, Di Sanzo G, Martino M, Casella P, Chianese S, Musmarra D (2018). Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent.Mar drugs 16 (11):432
Acknowledgements
AA is thankful to the CEFIPRA Indo French project for Pre doctoral fellowships. MJK is grateful to Department of Science and Technology Nanomission Government of India for the Post doc fellowship.
Funding
VV would like to thank Department of Science and Technology Nanomission Government of India research project number (SR/NM/NT-1090/2014(G) and Indo-French Centre for the Promotion of Advanced Research (IFCPAR/CEFIPRA) project number (PPMB-7133/2020) Indo France project sanctioned to VV for the financial aids.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ankesh Ahirwar, Mohd Jahir Khan, and Vandana Sirotiya are first co-authors.
Rights and permissions
About this article
Cite this article
Ahirwar, A., Khan, M.J., Sirotiya, V. et al. Pulsed Electric Field–Assisted Cell Permeabilization of Microalgae (Haematococcus pluvialis) for Milking of Value-Added Compounds. Bioenerg. Res. 16, 311–324 (2023). https://doi.org/10.1007/s12155-022-10414-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10414-4