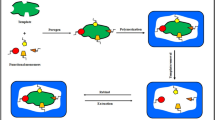
Abstract
At present, huge attention is implanted on the exploration and distribution of endemic plant biomass, which has dietary flavonoids in different food and livestock supplements manufacturing industries. Dietary flavonoids present in the plant biomass have wide phytotherapeutic properties such as antioxidant, anti-bacterial, anti-viral, and immuno-modulatory. This review aims to analyze and discuss various solvent-based extraction techniques (microwave-assisted extraction, subcritical extraction, and ultrasound-assisted extraction), optimum point and yield of flavonoids from various plant biomass. Furthermore, this article provides a review on separation and purification of flavonoids present in the complex matrix, using various solid-phase extraction (SPE) materials such as C-18 silica nanomaterials and molecularly imprinted polymers (MIPs) and molecularly imprinted membranes (MIMs). Solid-phase extraction (SPE) has been used to quickly analyze the trace elements which are present in the sample matrices network. MIPs and MIMs with suitable recognition sites are employed for the pre-treatment of samples before quantification. The SPE sorbent synthesizing protocol, interactions, characteristics, and effects are discussed. Furthermore, essential performance such as sorption, recovery ratio, and imprinting factor was evaluated based on previous research studies. Together with these, the overall polymerization mechanism of molecularly imprinted materials using monomers, initiator, and cross-linker for the selective separation of dietary flavonoids has been reviewed. Current trends in the molecularly imprinted polymers (MIPs) and molecularly imprinted membranes (MIMs) for effective separation of dietary flavonoids from plant biomass are discussed.
Graphical abstract











Similar content being viewed by others
References
Anagnostopoulou MA, Kefalas P, Papageorgiou VP et al (2006) Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem 94:19–25. https://doi.org/10.1016/j.foodchem.2004.09.047
Barrera-Garcí VD, Gougeon RD, Karbowiak T et al (2008) Role of Wood Macromolecules on Selective Sorption of Phenolic Compounds by Wood. J Agric Food Chem 56:8498–8506. https://doi.org/10.1021/JF801314N
Fernández-Agulló A, Freire MS, Ramírez-López C et al (2021) Valorization of residual walnut biomass from forest management and wood processing for the production of bioactive compounds. Biomass Convers Biorefinery 11:609–618. https://doi.org/10.1007/s13399-019-00598-9
García-Araya JF, Beltrán FJ, Álvarez P, Masa FJ (2003) Activated carbon adsorption of some phenolic compounds present in agroindustrial wastewater. Adsorption 9:107–115. https://doi.org/10.1023/A:1024228708675
Li J, Chase HA (2010) Development of adsorptive (non-ionic) macroporous resins and their uses in the purification of pharmacologically-active natural products from plant sources. Nat Prod Rep 27:1493–1510. https://doi.org/10.1039/C0NP00015A
Satchanska G (2022) Antibacterial activity of plant polyphenols. In: Vijayakumar R, Raja SSS (eds) Secondary metabolites - trends and reviews. IntechOpen, London. https://doi.org/10.5772/intechopen.101664
Kang J, Xie C, Li Z et al (2011) Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem 128:152–157. https://doi.org/10.1016/j.foodchem.2011.03.011
Rusanov K, Garo E, Rusanova M et al (2014) Recovery of Polyphenols from Rose Oil Distillation Wastewater Using Adsorption Resins – A Pilot Study. Planta Med 80:1657–1664. https://doi.org/10.1055/S-0034-1383145
Ma YQ, Chen JC, Liu DH, Ye XQ (2008) Effect of ultrasonic treatment on the total phenolic and antioxidant activity of extracts from citrus peel. J Food Sci 73. https://doi.org/10.1111/j.1750-3841.2008.00908.x
Tungmunnithum D, Drouet S, Lorenzo JM (2021) Hano C (2021) Green Extraction of Antioxidant Flavonoids from Pigeon Pea (Cajanus cajan (L.) Millsp.) Seeds and Its Antioxidant Potentials Using Ultrasound-Assisted Methodology. Mol 26:7557. https://doi.org/10.3390/MOLECULES26247557
Ignat I, Volf I, Popa VI (2011) A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem 126:1821–1835. https://doi.org/10.1016/J.FOODCHEM.2010.12.026
Sergent T, Piront N, Meurice J et al (2010) Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem Biol Interact 188:659–667. https://doi.org/10.1016/j.cbi.2010.08.007
Snyder SM, Reber JD, Freeman BL et al (2011) Controlling for sugar and ascorbic acid, a mixture of flavonoids matching navel oranges significantly increases human postprandial serum antioxidant capacity. Nutr Res 31:519–526. https://doi.org/10.1016/j.nutres.2011.06.006
Barros RGC, Andrade JKS, Denadai M et al (2017) Evaluation of bioactive compounds potential and antioxidant activity in some Brazilian exotic fruit residues. Food Res Int 102:84–92. https://doi.org/10.1016/j.foodres.2017.09.082
Gan CY, Latiff AA (2011) Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem 124:1277–1283. https://doi.org/10.1016/j.foodchem.2010.07.074
Prakash Maran J, Manikandan S, Vigna Nivetha C, Dinesh R (2017) Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab J Chem 10:S1145–S1157. https://doi.org/10.1016/j.arabjc.2013.02.007
de Elguea-Culebras GO, Bravo EM, Sánchez-Vioque R (2022) Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of Mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market – A review. Ind Crops Prod 175:114261. https://doi.org/10.1016/j.indcrop.2021.114261
Michalkiewicz A, Biesaga M, Pyrzynska K (2008) Solid-phase extraction procedure for determination of phenolic acids and some flavonols in honey. J Chromatogr A 1187:18–24. https://doi.org/10.1016/J.CHROMA.2008.02.001
Li X, Chen F, Li S et al (2016) An efficient homogenate-microwave-assisted extraction of flavonols and anthocyanins from blackcurrant marc: Optimization using combination of Plackett-Burman design and Box-Behnken design. Ind Crops Prod 94:834–847. https://doi.org/10.1016/j.indcrop.2016.09.063
Chidambara Murthy KN, Jayaprakasha GK, Singh RP (2002) Studies on Antioxidant Activity of Pomegranate ( Punica granatum ) Peel Extract Using in Vivo Models. J Agric Food Chem 50:4791–4795. https://doi.org/10.1021/jf0255735
Koyu H, Kazan A, Demir S et al (2018) Optimization of microwave assisted extraction of Morus nigra L. fruits maximizing tyrosinase inhibitory activity with isolation of bioactive constituents. Food Chem 248:183–191. https://doi.org/10.1016/j.foodchem.2017.12.049
Karabegović IT, Stojičević SS, Veličković DT et al (2013) Optimization of microwave-assisted extraction and characterization of phenolic compounds in cherry laurel (Prunus laurocerasus) leaves. Sep Purif Technol 120:429–436. https://doi.org/10.1016/j.seppur.2013.10.021
Jesus MS, Genisheva Z, Romaní A et al (2019) Bioactive compounds recovery optimization from vine pruning residues using conventional heating and microwave-assisted extraction methods. Ind Crops Prod 132:99–110. https://doi.org/10.1016/j.indcrop.2019.01.070
Pinela J, Prieto MA, Carvalho AM et al (2016) Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep Purif Technol 164:114–124. https://doi.org/10.1016/j.seppur.2016.03.030
Zheng X, Xu X, Liu C et al (2013) Extraction characteristics and optimal parameters of anthocyanin from blueberry powder under microwave-assisted extraction conditions. Sep Purif Technol 104:17–25. https://doi.org/10.1016/j.seppur.2012.11.011
Kaderides K, Papaoikonomou L, Serafim M, Goula AM (2019) Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem Eng Process - Process Intensif 137:1–11. https://doi.org/10.1016/j.cep.2019.01.006
Kırbaşlar Şİ, Şahin S (2021) Recovery of bioactive ingredients from biowaste of olive tree (Olea europaea) using microwave-assisted extraction: a comparative study. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01194-y
Xie J, Zhu L, Luo H et al (2001) Direct extraction of specific pharmacophoric flavonoids from gingko leaves using a molecularly imprinted polymer for quercetin. J Chromatogr A 934:1–11. https://doi.org/10.1016/S0021-9673(01)01294-8
Zhong H, Marcus SL, Li L (2005) Microwave-assisted acid hydrolysis of proteins combined with liquid chromatography MALDI MS/MS for protein identification. J Am Soc Mass Spectrom 16:471–481. https://doi.org/10.1016/j.jasms.2004.12.017
Xiping Chen J, Zhang Y (2008) Rapid microwave-assisted hydrolysis for determination of ginkgo flavonol glycosides in extracts of Ginkgo biloba leaves. J Chromatogr Sci 46:117–121. https://doi.org/10.1093/chromsci/46.2.117
Madej K (2009) Microwave-assisted and cloud-point extraction in determination of drugs and other bioactive compounds. TrAC - Trends Anal Chem 28:436–446. https://doi.org/10.1016/j.trac.2009.02.002
Mandal V, Mohan Y, Hemalatha S et al (2007) Microwave assisted extraction-an innovative and promising extraction tool for medicinal plant research. Pharmacogn Rev 1(1):7–18
Proestos C, Komaitis M (2008) Application of microwave-assisted extraction to the fast extraction of plant phenolic compounds. LWT - Food Sci Technol 41:652–659. https://doi.org/10.1016/j.lwt.2007.04.013
Kumar BB, Smita K, Kumar BB et al (2014) Microwave-Assisted Extraction and Solid-Phase Separation of Quercetin from Solid Onion (Allium cepa L.). Sep Sci Technol 49:2502–2509. https://doi.org/10.1080/01496395.2014.933982
Chaves JO, de Souza MC, da Silva LC et al (2020) Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front Chem 8:864. https://doi.org/10.3389/FCHEM.2020.507887/BIBTEX
Pandey S (2006) Analytical applications of room-temperature ionic liquids: A review of recent efforts. Anal Chim Acta 556:38–45. https://doi.org/10.1016/j.aca.2005.06.038
Zhang DH, Bai S, Ren MY, Sun Y (2008) Optimization of lipase-catalyzed enantioselective esterification of (±)-menthol in ionic liquid. Food Chem 109:72–80. https://doi.org/10.1016/j.foodchem.2007.12.020
Kim MJ, Choi MY, Lee JK, Ahn Y (2003) Enzymatic selective acylation of glycosides in ionic liquids: Significantly enhanced reactivity and regioselectivity. J Mol Catal B Enzym 26:115–118. https://doi.org/10.1016/j.molcatb.2003.04.001
Polyakova Y, Jin Y, Zheng J, Ho Row K (2006) Effect of concentration of ionic liquid 1-butyl-3-methylimidazolium, tetrafuoroborate, for retention and separation of some amino and nucleic acids. J Liq Chromatogr Relat Technol 29:1687–1701. https://doi.org/10.1080/10826070600716769
Armstrong DW, He L, Liu YS (1999) Examination of ionic liquids and their interaction with molecules, when used as stationary phases in gas chromatography. Anal Chem 71:3873–3876. https://doi.org/10.1021/ac990443p
Qiu H, Jiang S, Liu X (2006) N-Methylimidazolium anion-exchange stationary phase for high-performance liquid chromatography. J Chromatogr A 1103:265–270. https://doi.org/10.1016/j.chroma.2005.11.035
Kokosa JM (2019) Selecting an extraction solvent for a greener liquid phase microextraction (LPME) mode-based analytical method. TrAC - Trends Anal Chem 118:238–247. https://doi.org/10.1016/J.TRAC.2019.05.012
Du FY, Xiao XH, Li GK (2011) Ionic liquid aqueous solvent-based microwave-assisted hydrolysis for the extraction and HPLC determination of myricetin and quercetin from Myrica rubra leaves. Biomed Chromatogr 25:472–478. https://doi.org/10.1002/bmc.1470
Mustapa AN, Martin Á, Mato RB, Cocero MJ (2015) Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind Crops Prod 74:83–94. https://doi.org/10.1016/j.indcrop.2015.04.035
Talmaciu AI, Ravber M, Volf I et al (2016) Isolation of bioactive compounds from spruce bark waste using sub- and supercritical fluids. J Supercrit Fluids 117:243–251. https://doi.org/10.1016/j.supflu.2016.07.001
Rafiee Z, Jafari SM, Alami M, Khomeiri M (2011) Microwave-assisted extraction of phenolic compounds from olive leaves; a comparison with maceration. J Anim Plant Sci 21:738–745
Pan X, Niu G, Liu H (2003) Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Process 42:129–133. https://doi.org/10.1016/S0255-2701(02)00037-5
Munir MT, Kheirkhah H, Baroutian S et al (2018) Subcritical water extraction of bioactive compounds from waste onion skin. J Clean Prod 183:487–494. https://doi.org/10.1016/j.jclepro.2018.02.166
Erşan S, Güçlü Üstündağ Ö, Carle R, Schweiggert RM (2018) Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem 253:46–54. https://doi.org/10.1016/j.foodchem.2018.01.116
Plaza M, Amigo-Benavent M, del Castillo MD et al (2010) Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res Int 43:2341–2348. https://doi.org/10.1016/j.foodres.2010.07.036
Ko MJ, Cheigh CI, Cho SW, Chung MS (2011) Subcritical water extraction of flavonol quercetin from onion skin. J Food Eng 102:327–333. https://doi.org/10.1016/j.jfoodeng.2010.09.008
Kim SW, Ko MJ, Chung MS (2019) Extraction of the flavonol quercetin from onion waste by combined treatment with intense pulsed light and subcritical water extraction. J Clean Prod 231:1192–1199. https://doi.org/10.1016/j.jclepro.2019.05.280
Hartonen K, Parshintsev J, Sandberg K et al (2007) Isolation of flavonoids from aspen knotwood by pressurized hot water extraction and comparison with other extraction techniques. Talanta 74:32–38. https://doi.org/10.1016/j.talanta.2007.05.040
Kumar MSY, Dutta R, Prasad D, Misra K (2011) Subcritical water extraction of antioxidant compounds from Seabuckthorn (Hippophae rhamnoides) leaves for the comparative evaluation of antioxidant activity. Food Chem 127:1309–1316. https://doi.org/10.1016/j.foodchem.2011.01.088
Ibañez E, Kubátová A, Señoráns FJ et al (2003) Subcritical water extraction of antioxidant compounds from rosemary plants. J Agric Food Chem 51:375–382. https://doi.org/10.1021/jf025878j
Rodríguez-Meizoso I, Marin FR, Herrero M et al (2006) Subcritical water extraction of nutraceuticals with antioxidant activity from oregano. Chemical and functional characterization. J Pharm Biomed Anal 41:1560–1565. https://doi.org/10.1016/j.jpba.2006.01.018
Essien S, Young B, Baroutian S (2020) Subcritical water extraction for selective recovery of phenolic bioactives from kānuka leaves. J Supercrit Fluids 158:5–7. https://doi.org/10.1016/j.supflu.2019.104721
Reis SF, Rai DK, Abu-Ghannam N (2012) Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chem. https://doi.org/10.1016/j.foodchem.2012.06.068
Aliakbarian B, Fathi A, Perego P, Dehghani F (2012) Extraction of antioxidants from winery wastes using subcritical water. J Supercrit Fluids 65:18–24. https://doi.org/10.1016/j.supflu.2012.02.022
Dailey A, Vuong QV (2015) Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric 1. https://doi.org/10.1080/23311932.2015.1115646
Yang Y, Belghazi M, Lagadec A et al (1998) Elution of organic solutes from different polarity sorbents using subcritical water. J Chromatogr A 810:149–159. https://doi.org/10.1016/S0021-9673(98)00222-2
Rodríguez-Rojo S, Visentin A, Maestri D, Cocero MJ (2012) Assisted extraction of rosemary antioxidants with green solvents. J Food Eng 109:98–103. https://doi.org/10.1016/j.jfoodeng.2011.09.029
Munekata PES, Alcántara C, Žugčić T et al (2020) Impact of ultrasound-assisted extraction and solvent composition on bioactive compounds and in vitro biological activities of thyme and rosemary. Food Res Int 134. https://doi.org/10.1016/j.foodres.2020.109242
Ahmad-Qasem MH, Cánovas J, Barrajón-Catalán E et al (2013) Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov Food Sci Emerg Technol 17:120–129. https://doi.org/10.1016/j.ifset.2012.11.008
Barba FJ, Grimi N, Vorobiev E (2015) Evaluating the potential of cell disruption technologies for green selective extraction of antioxidant compounds from Stevia rebaudiana Bertoni leaves. J Food Eng 149:222–228. https://doi.org/10.1016/j.jfoodeng.2014.10.028
Gavahian M, Farhoosh R, Javidnia K, et al (2017) Effects of Electrolyte Concentration and Ultrasound Pretreatment on Ohmic-Assisted Hydrodistillation of Essential Oils from Mentha piperita L. Int J Food Eng 13. https://doi.org/10.1515/ijfe-2017-0010
Khemakhem I, Ahmad-Qasem MH, Catalán EB et al (2017) Kinetic improvement of olive leaves’ bioactive compounds extraction by using power ultrasound in a wide temperature range. Ultrason Sonochem 34:466–473. https://doi.org/10.1016/j.ultsonch.2016.06.010
Roselló-Soto E, Galanakis CM, Brnčić M et al (2015) Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci Technol 42:134–149. https://doi.org/10.1016/j.tifs.2015.01.002
Asfaram A, Ghaedi M, Javadian H, Goudarzi A (2018) Cu- and S- @SnO2 nanoparticles loaded on activated carbon for efficient ultrasound assisted dispersive µSPE-spectrophotometric detection of quercetin in Nasturtium officinale extract and fruit juice samples: CCD-RSM design. Ultrason Sonochem 47:1–9. https://doi.org/10.1016/j.ultsonch.2018.04.008
Sánchez-Vioque R, Polissiou M, Astraka K et al (2013) Polyphenol composition and antioxidant and metal chelating activities of the solid residues from the essential oil industry. Ind Crops Prod 49:150–159. https://doi.org/10.1016/J.INDCROP.2013.04.053
Goltz C, Ávila S, Barbieri JB et al (2018) Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. Ind Crops Prod 115:227–234. https://doi.org/10.1016/j.indcrop.2018.02.013
Xu DP, Zheng J, Zhou Y et al (2017) Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem 217:552–559. https://doi.org/10.1016/j.foodchem.2016.09.013
Ribeiro DN, Alves FMS, dos Santos Ramos VH et al (2020) Extraction of passion fruit (Passiflora cincinnata Mast.) pulp oil using pressurized ethanol and ultrasound: Antioxidant activity and kinetics. J Supercrit Fluids 165:104944. https://doi.org/10.1016/j.supflu.2020.104944
Madrera RR, Valles BS (2020) Development and validation of ultrasound assisted extraction (UAE) and HPLC-DAD method for determination of polyphenols in dry beans (Phaseolus vulgaris). J Food Compos Anal 85:103334. https://doi.org/10.1016/j.jfca.2019.103334
Santos MC, Koetz M, Mendez ASL, Henriques AT (2020) Ultrasound-assisted extraction optimization and validation of ultra-performance liquid chromatographic method for the quantification of miquelianin in Cuphea glutinosa leaves. Talanta 216. https://doi.org/10.1016/j.talanta.2020.120988
Radziejewska-Kubzdela E, Szwengiel A, Ratajkiewicz H, Nowak K (2020) Effect of ultrasound, heating and enzymatic pre-treatment on bioactive compounds in juice from Berberis amurensis Rupr. Ultrason Sonochem 63:104971. https://doi.org/10.1016/j.ultsonch.2020.104971
Xiao Y, Zhang H (2012) Homogeneous ionic liquid microextraction of the active constituents from fruits of Schisandra chinensis and Schisandra sphenanthera. Anal Chim Acta 712:78–84. https://doi.org/10.1016/j.aca.2011.11.033
Chemat F, Rombaut N, Sicaire AG et al (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review Ultrason Sonochem 34:540–560. https://doi.org/10.1016/j.ultsonch.2016.06.035
Corbin C, Fidel T, Leclerc EA et al (2015) Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason Sonochem 26:176–185. https://doi.org/10.1016/j.ultsonch.2015.02.008
Nayak B, Dahmoune F, Moussi K, Remini H, Dairi S, Aoun O, Khodir M et al (2015) Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem 187:507–516. https://doi.org/10.1016/j.foodchem.2015.04.081
Dahmoune F, Boulekbache L, Moussi K et al (2013) Valorization of Citrus limon residues for the recovery of antioxidants: Evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind Crops Prod 50:77–87. https://doi.org/10.1016/j.indcrop.2013.07.013
Sparr Eskilsson C, Björklund E (2000) Analytical-scale microwave-assisted extraction. J Chromatogr A 902:227–250. https://doi.org/10.1016/S0021-9673(00)00921-3
Chen XJ, Guo BL, Li SP et al (2007) Simultaneous determination of 15 flavonoids in Epimedium using pressurized liquid extraction and high-performance liquid chromatography. J Chromatogr A 1163:96–104. https://doi.org/10.1016/j.chroma.2007.06.020
Tahmasebi E, Yamini Y (2012) Facile synthesis of new nano sorbent for magnetic solid-phase extraction by self assembling of bis-(2,4,4-trimethyl pentyl)-dithiophosphinic acid on Fe3O4@Ag core@shell nanoparticles: Characterization and application. Anal Chim Acta 756:13–22. https://doi.org/10.1016/j.aca.2012.10.040
Vidal L, Parshintsev J, Hartonen K et al (2012) Ionic liquid-functionalized silica for selective solid-phase extraction of organic acids, amines and aldehydes. J Chromatogr A 1226:2–10. https://doi.org/10.1016/j.chroma.2011.08.075
Liu H, Liang X, Wang X et al (2015) Polyelectrolyte assembled graphene oxide coated silica composite as sorbent for solid-phase extraction of cinnamic acid and its derivatives. RSC Adv 5:4420–4427. https://doi.org/10.1039/c4ra13331e
Guo F, Liu Q, Qu G-b et al (2013) Simultaneous determination of five estrogens and four androgens in water samples by online solid-phase extraction coupled with high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1281:9–18. https://doi.org/10.1016/j.chroma.2013.01.044
Liu H, Zhang M, Guo Y, Qiu H (2016) Solid-phase extraction of flavonoids in honey samples using carbamate-embedded triacontyl-modified silica sorbent. Food Chem 204:56–61. https://doi.org/10.1016/j.foodchem.2016.02.102
Bagheri A, Behbahani M, Taghizadeh M et al (2012) Polymer As a New Sorbent for Selective Extraction and Preconcentration of. Food Chem. https://doi.org/10.1016/j.foodchem.2012.11.042
Liu H, Guo Y, Wang X et al (2015) Preparation of an Al2O3/SiO2 core-shell composite material for solid phase extraction of flavonoids. Anal Methods 7:3486–3492. https://doi.org/10.1039/c5ay00271k
Pardo A, Mespouille L, Blankert B et al (2014) Quercetin-imprinted chromatographic sorbents revisited: Optimization of synthesis and rebinding protocols for application to natural resources. J Chromatogr A 1364:128–139. https://doi.org/10.1016/j.chroma.2014.08.064
Nam MW, Zhao J, Lee MS et al (2015) Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem 17:1718–1727. https://doi.org/10.1039/c4gc01556h
Wang Y, Gao S, Zang X et al (2012) Graphene-based solid-phase extraction combined with flame atomic absorption spectrometry for a sensitive determination of trace amounts of lead in environmental water and vegetable samples. Anal Chim Acta 716:112–118. https://doi.org/10.1016/j.aca.2011.12.007
Tian M, Qiao J, Row KH (2013) Facile Preparation of an Ionic Liquid Composite Mesoporous Polymer as a Solid Phase Extraction Adsorbent for the Separation and Purification of Flavonoids from Chamaecyparis obtusa. Anal Lett 46:1331–1341. https://doi.org/10.1080/00032719.2012.763170
Taylor P, Rao TP, Praveen RS et al (2010) Critical Reviews in Analytical Chemistry Styrene – Divinyl Benzene Copolymers : Synthesis , Characterization , and Their Role in Inorganic Trace Analysis Styrene – Divinyl Benzene Copolymers : Synthesis , Characterization , and Their Role in Inorganic Tra. 37–41. https://doi.org/10.1080/10408340490888689
Lemos VA, Santos MS, Santos ES et al (2007) Application of polyurethane foam as a sorbent for trace metal pre-concentration — a review. Spectrochimica Acta Part B 62:4–12. https://doi.org/10.1016/j.sab.2006.12.006
Wang M, Yuan H, Deng W et al (2015) A Taiji-principle-designed magnetic porous C-doped graphitic carbon nitride for environment-friendly solid phase extraction of pollutants from water samples. J Chromatogr A 1412:12–21. https://doi.org/10.1016/j.chroma.2015.08.011
Hao L, Wang C, Wu Q et al (2014) Metal-organic framework derived magnetic nanoporous carbon: Novel adsorbent for magnetic solid-phase extraction. Anal Chem 86:12199–12205. https://doi.org/10.1021/ac5031896
Shi R, Yan L, Xu T et al (2015) Graphene oxide bound silica for solid-phase extraction of 14 polycyclic aromatic hydrocarbons in mainstream cigarette smoke. J Chromatogr A 1375:1–7. https://doi.org/10.1016/j.chroma.2014.11.057
Feng J, Sun M, Li J et al (2012) A novel aromatically functional polymeric ionic liquid as sorbent material for solid-phase microextraction. J Chromatogr A 1227:54–59. https://doi.org/10.1016/j.chroma.2012.01.010
Feng J, Sun M, Bu Y, Luo C (2015) Facile modification of multi-walled carbon nanotubes-polymeric ionic liquids-coated solid-phase microextraction fibers by on-fiber anion exchange. J Chromatogr A 1393:8–17. https://doi.org/10.1016/j.chroma.2015.03.022
Qiu H, Mallik AK, Takafuji M et al (2012) Enhancement of molecular shape selectivity by in situ anion-exchange in poly(octadecylimidazolium) silica column. J Chromatogr A 1232:116–122. https://doi.org/10.1016/j.chroma.2011.10.065
Ghiasvand AR, Solaymani H, Heidari N (2017) Separation and sensitive determination of quercetin in Rosa canina L. using solidified floating organic drop microextraction followed by high-performance liquid chromatography determination. J Iran Chem Soc 14:1113–1118. https://doi.org/10.1007/s13738-017-1061-9
Wang Z, Sun R, Wang Y et al (2014) Determination of phenolic acids and flavonoids in raw propolis by silica-supported ionic liquid-based matrix solid phase dispersion extraction high performance liquid chromatography-diode array detection. J Chromatogr B Anal Technol Biomed Life Sci 969:205–212. https://doi.org/10.1016/j.jchromb.2014.08.022
Hou X, Liu S, Zhou P et al (2016) Polymeric ionic liquid modified graphene oxide-grafted silica for solid-phase extraction to analyze the excretion-dynamics of flavonoids in urine by Box-Behnken statistical design. J Chromatogr A 1456:10–18. https://doi.org/10.1016/j.chroma.2016.05.096
Krňanová J, Denderz N, Lehotay J, Samohýl M (2015) Determination of some flavonoids by HPLC using quercetin-molecularly imprinted polymers. J Liq Chromatogr Relat Technol 38:702–708. https://doi.org/10.1080/10826076.2014.951768
Martins RO, Gomes IC, Mendonça Telles AD et al (2020) Molecularly imprinted polymer as solid phase extraction phase for condensed tannin determination from Brazilian natural sources. J Chromatogr A 460977. https://doi.org/10.1016/j.chroma.2020.460977
Euterpio MA, Pagano I, Piccinelli AL et al (2013) Development and validation of a method for the determination of (E)-resveratrol and related phenolic compounds in beverages using molecularly imprinted solid phase extraction. J Agric Food Chem 61:1640–1645. https://doi.org/10.1021/jf303251m
Ji K, Luo X, He L et al (2020) Preparation of hollow magnetic molecularly imprinted polymer and its application in silybin recognition and controlled release. J Pharm Biomed Anal 180:113036. https://doi.org/10.1016/j.jpba.2019.113036
Cheng Y, Nie J, Li J et al (2019) Synthesis and characterization of core–shell magnetic molecularly imprinted polymers for selective recognition and determination of quercetin in apple samples. Food Chem 287:100–106. https://doi.org/10.1016/j.foodchem.2019.02.069
Wulff G (2013) Fourty years of molecular imprinting in synthetic polymers: Origin, features and perspectives. Microchim Acta 180:1359–1370. https://doi.org/10.1007/s00604-013-0992-9
Samah NA, Sánchez-Martín M-J, Sebastián RM et al (2018) Molecularly imprinted polymer for the removal of diclofenac from water: Synthesis and characterization. Sci Total Environ 631–632:1534–1543. https://doi.org/10.1016/j.scitotenv.2018.03.087
Ulbricht M (2004) Membrane separations using molecularly imprinted polymers. J Chromatogr B Anal Technol Biomed Life Sci 804:113–125. https://doi.org/10.1016/j.jchromb.2004.02.007
Kryvshenko GA, Apel PY, Abramchuk SS, Beklemishev MK (2012) A Highly Permeable Membrane for Separation of Quercetin Obtained by Nickel(II) Ion-Mediated Molecular Imprinting. Sep Sci Technol 47:1715–1724. https://doi.org/10.1080/01496395.2012.659317
Piletsky SA, Panasyuk TL, Piletskaya EV et al (1999) Receptor and transport properties of imprinted polymer membranes - A review. J Memb Sci 157:263–278. https://doi.org/10.1016/S0376-7388(99)00007-1
Theodoridis G, Lasáková M, Škeříková V et al (2006) Molecular imprinting of natural flavonoid antioxidants: Application in solid-phase extraction for the sample pretreatment of natural products prior to HPLC analysis. J Sep Sci 29:2310–2321. https://doi.org/10.1002/jssc.200500492
Chen C-Y, Wang C-H, Chen A-H (2011) Recognition of molecularly imprinted polymers for a quaternary alkaloid of berberine. Talanta 84:1038–1046. https://doi.org/10.1016/j.talanta.2011.03.009
Huang Y, Pan J, Liu Y et al (2019) A SPE method with two MIPs in two steps for improving the selectivity of MIPs. Anal Chem 91:8436–8442. https://doi.org/10.1021/acs.analchem.9b01453
Liang C, Zhang Z, Zhang H et al (2020) Ordered macroporous molecularly imprinted polymers prepared by a surface imprinting method and their applications to the direct extraction of flavonoids from Gingko leaves. Food Chem 309:125680. https://doi.org/10.1016/j.foodchem.2019.125680
Huber C, Preis M, Harvey PJ et al (2016) Emerging pollutants and plants - Metabolic activation of diclofenac by peroxidases. Chemosphere 146:435–441. https://doi.org/10.1016/j.chemosphere.2015.12.059
Chrzanowska AM, Poliwoda A, Wieczorek PP (2015) Characterization of particle morphology of biochanin A molecularly imprinted polymers and their properties as a potential sorbent for solid-phase extraction. Mater Sci Eng C 49:793–798. https://doi.org/10.1016/j.msec.2015.01.069
Guć M, Messyasz B, Schroeder G (2021) Environmental impact of molecularly imprinted polymers used as analyte sorbents in mass spectrometry. Sci Total Environ 772:145074. https://doi.org/10.1016/j.scitotenv.2021.145074
Mahony JO, Nolan K, Smyth MR, Mizaikoff B (2005) Molecularly imprinted polymers - Potential and challenges in analytical chemistry. Anal Chim Acta 534:31–39. https://doi.org/10.1016/J.ACA.2004.07.043
O’Mahony J, Molinelli A, Nolan K et al (2006) Anatomy of a successful imprint: Analysing the recognition mechanisms of a molecularly imprinted polymer for quercetin. Biosens Bioelectron 21:1383–1392. https://doi.org/10.1016/j.bios.2005.05.015
Zengin A, Badak MU, Aktas N (2018) Selective separation and determination of quercetin from red wine by molecularly imprinted nanoparticles coupled with HPLC and ultraviolet detection. J Sep Sci 41:3459–3466. https://doi.org/10.1002/jssc.201800437
Hemmati K, Masoumi A, Ghaemy M (2016) Tragacanth gum-based nanogel as a superparamagnetic molecularly imprinted polymer for quercetin recognition and controlled release. Carbohydr Polym 136:630–640. https://doi.org/10.1016/j.carbpol.2015.09.006
Zaidi SA, Shin JH (2014) Molecularly imprinted polymer electrochemical sensors based on synergistic effect of composites synthesized from graphene and other nanosystems. Int J Electrochem Sci 9:4598–4616
Molinelli A, Weiss R, Mizaikoff B (2002) Advanced solid phase extraction using molecularly imprinted polymers for the determination of quercetin in red wine. J Agric Food Chem 50:1804–1808. https://doi.org/10.1021/jf011213q
Song X, Li J, Wang J, Chen L (2009) Quercetin molecularly imprinted polymers: Preparation, recognition characteristics and properties as sorbent for solid-phase extraction. Talanta 80:694–702. https://doi.org/10.1016/j.talanta.2009.07.051
Karaman Ersoy Ş, Tütem E, Sözgen Başkan K et al (2016) Preparation, characterization and usage of molecularly imprinted polymer for the isolation of quercetin from hydrolyzed nettle extract. J Chromatogr B Anal Technol Biomed Life Sci 1017–1018:89–100. https://doi.org/10.1016/j.jchromb.2016.02.034
Pakade V, Cukrowska E, Lindahl S et al (2013) Molecular imprinted polymer for solid-phase extraction of flavonol aglycones from Moringa oleifera extracts. J Sep Sci 36:548–555. https://doi.org/10.1002/jssc.201200576
Gao B, Niu Q, Du R (2010) Preparation and recognition performance of cytisine alkaloid-imprinted material prepared using novel surface molecular imprinting technique. J Sep Sci 33:1338–1348. https://doi.org/10.1002/jssc.200900762
Singh B, Chauhan N, Sharma V (2011) Design of molecular imprinted hydrogels for controlled release of cisplatin: Evaluation of network density of hydrogels. Ind Eng Chem Res 50:13742–13751. https://doi.org/10.1021/ie200758b
Dramou P, Itatahine A, Fizir M et al (2019) Preparation of novel molecularly imprinted magnetic graphene oxide and their application for quercetin determination. J Chromatogr B Anal Technol Biomed Life Sci 1124:273–283. https://doi.org/10.1016/j.jchromb.2019.06.007
Xie L, Guo J, Zhang Y, Shi S (2014) Efficient determination of protocatechuic acid in fruit juices by selective and rapid magnetic molecular imprinted solid phase extraction coupled with HPLC. J Agric Food Chem 62:8221–8228. https://doi.org/10.1021/jf5021895
Marć M, Wieczorek PP (2020) The preparation and evaluation of core-shell magnetic dummy-template molecularly imprinted polymers for preliminary recognition of the low-mass polybrominated diphenyl ethers from aqueous solutions. Sci Total Environ 724:138151. https://doi.org/10.1016/j.scitotenv.2020.138151
Singh R, Verma R, Sumana G et al (2012) Nanobiocomposite platform based on polyaniline-iron oxide-carbon nanotubes for bacterial detection. Bioelectrochemistry 86:30–37. https://doi.org/10.1016/j.bioelechem.2012.01.005
Wang J, Geng S, Wang B et al (2017) Magnetic nanoparticles and high-speed countercurrent chromatography coupled in-line and using the same solvent system for separation of quercetin-3-O-rutinoside, luteoloside and astragalin from a Mikania micrantha extract. J Chromatogr A 1508:42–52. https://doi.org/10.1016/j.chroma.2017.05.062
Hemmati K, Sahraei R, Ghaemy M (2016) Synthesis and characterization of a novel magnetic molecularly imprinted polymer with incorporated graphene oxide for drug delivery. Polymer (Guildf) 101:257–268. https://doi.org/10.1016/j.polymer.2016.08.074
Sabín López A, Paredes Ramos M, Herrero R, López Vilariño JM (2020) Synthesis of magnetic green nanoparticle – Molecular imprinted polymers with emerging contaminants templates. J Environ Chem Eng 8:103889. https://doi.org/10.1016/j.jece.2020.103889
Faizal CKM, Hoshina Y, Kobayashi T (2008) Scaffold membranes for selective adsorption of α-tocopherol by phase inversion covalently imprinting technique. J Memb Sci 322:503–511. https://doi.org/10.1016/j.memsci.2008.05.046
Tasselli F, Donato L, Drioli E (2008) Evaluation of molecularly imprinted membranes based on different acrylic copolymers. J Memb Sci 320:167–172. https://doi.org/10.1016/j.memsci.2008.03.071
Steinke J, Sherrington DC, Dunkin IR (1995) Imprinting of synthetic polymers using molecular templates. Adv Polym Sci 123:81–125. https://doi.org/10.1007/3-540-58908-2_3
Wang J, Hua ZD, Chen ZY et al (2009) Molecular imprinting of protein by coordinate interaction. Chinese Chem Lett 20:747–750. https://doi.org/10.1016/j.cclet.2008.12.035
Papaioannou EH, Liakopoulou-Kyriakides M, Papi RM, Kyriakidis DA (2008) Artificial receptor for peptide recognition in protic media: The role of metal ion coordination. Mater Sci Eng B Solid-State Mater Adv Technol 152:28–32. https://doi.org/10.1016/j.mseb.2008.06.017
Zheng MX, Li SJ, Luo X (2007) Rationally designing molecularly imprinted polymer toward a high specific adsorbent by using metal as assembled pivot. J Macromol Sci Part A Pure Appl Chem 44:1187–1194. https://doi.org/10.1080/10601320701561122
Shan J, Wang B (2011) Preparation and characterization of a metal-complexing imprinted polymer for improved Quercetin recognition. Sep Sci Technol 46:164–171. https://doi.org/10.1080/01496391003789189
Esfandyari-Manesh M, Javanbakht M, Atyabi F et al (2011) Effect of porogenic solvent on the morphology, recognition and release properties of carbamazepine-molecularly imprinted polymer nanospheres. J Appl Polym Sci 121:1118–1126. https://doi.org/10.1002/app.33812
Iben Nasser I, Algieri C, Garofalo A et al (2016) Hybrid imprinted membranes for selective recognition of quercetin. Sep Purif Technol 163:331–340. https://doi.org/10.1016/j.seppur.2016.03.015
Huang Z, Zhang P, Yun Y (2017) Preparing molecularly imprinted membranes by phase inversion to separate kaempferol. Polym Adv Technol 28:373–378. https://doi.org/10.1002/pat.3898
Huang Z, Xia Q, Yun Y (2018) Effects of different organic additives on kaempferol molecularly imprinted membrane properties. Polym Bull 75:441–452. https://doi.org/10.1007/s00289-017-2044-9
Acknowledgements
The authors would like to thank the Department of Science and Technology, India, for providing financial support (INT/HUN/P-17/2017); the Hungarian Science and Research Foundation under Grant 2017–2.3.7-TÉT–IN–2017-00016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We proclaim no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gokulakrishnan, S.A., Arthanareeswaran, G., Gnanasekaran, G. et al. Advanced extraction and separation approaches for the recovery of dietary flavonoids from plant biomass: A review. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-02648-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-02648-1