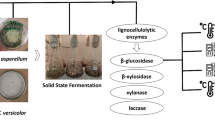
Abstract
Agro-industrial residues have progressed from an environmental concern toward sustainable and cost-effective sources of biopolymers and value-added chemicals. However, pre-treatments are often required for efficient extraction of the desired products from these residues. Fungal biorefinery is an interesting approach for the bioconversion of various biomasses into multiple products in a single batch. This study focuses on the utilization of six agro-industrial residues (grape pomace, sugarcane bagasse, rice husks, spent coffee grounds, residual diatomaceous earth, and wastepaper) in statistically designed combinations to evaluate the production of enzymes and the release of reducing sugars and phenolic compounds by the fungi Trametes villosa and Pycnoporus sanguineus, inoculated as mycelial pellets or mycelial discs. Both fungi demonstrated concurrent production of cellulases, pectinases, and laccases in semi-solid media, releasing sugars and phenolics from the substrates. However, each fungus exhibited distinct responses to different media compositions. P. sanguineus was the best cellulase producer, achieving a filter paper activity of 0.58 U mL−1 when inoculated as mycelial discs. T. villosa, on the other hand, excelled in producing pectinases (7.58 U mL−1 as mycelial discs) and laccases (1438 U L−1 as mycelial pellets). The highest concentrations of reducing sugars and phenolic compounds were found in extracts derived from P. sanguineus mycelial discs (37.12 mg mL−1) and T. villosa mycelial discs (0.261 mg GAE mL−1), respectively. Grape pomace and the addition of copper sulfate in the culture media consistently demonstrated their beneficial impact on the overall process.

Similar content being viewed by others
Data availability
Data supporting the findings of this study are available within its supplementary materials. Any additional data are available from the corresponding author G. M. M., upon reasonable request.
References
Dai Y, Sun Q, Wang W, Lu L, Liu M, Li J, Yang S, Sun Y, Zhang K, Xu J, Zheng W, Hu Z, Yang Y, Gao Y, Chen Y, Zhang X, Gao F, Zhang Y (2018) Utilizantions of agricultural waste as adsorbent for the removal of contaminants: a review. Chemosphere 211:235–253. https://doi.org/10.1016/j.chemosphere.2018.06.179
Geissdoerfer M, Savaget P, Bocken NMP, Hultink EJ (2017) The circular economy – a new sustainability paradigm? J Clean Prod 143:757–768. https://doi.org/10.1016/j.jclepro.2016.12.048
Tuysuz E, Gonul-Baltaci N, Omeroglu MA, Adiguzel A, Taskin M, Ozkan H (2020) Co-production of amylase and protease by locally isolated thermophilic bacterium Anoxybacillus rupiensis T2 in sterile and non-sterile media using waste potato peels as substrate. Waste Biomass Valorization 11(12):6793–6802. https://doi.org/10.1007/s12649-020-00936-3
Venkatesh G (2022) Circular bio-economy-paradigm for the future: systematic review of scientific journal publications from 2015 to 2021. Circ Econ Sust 2:231–279. https://doi.org/10.1007/s43615-021-00084-3
Brandão AS, Gonçalves A, Santos JMRCA (2021) Circular bioeconomy strategies: from scientific research to commercially viable products. J Clean Prod 295:126407. https://doi.org/10.1016/j.jclepro.2021.126407
Kuthiala T, Thakur K, Sharma D, Singh G, Khatri M, Arya SK (2022) The eco-friendly approach of cocktail enzyme in agricultural waste treatment: a comprehensive review. Int J Biol Macromol 209:1956–1974. https://doi.org/10.1016/j.ijbiomac.2022.04.173
Bilal M, Rasheed T, Iqbal HM, Yan Y (2018) Peroxidases-assisted removal of environmentally related hazardous pollutants with reference to the reaction mechanisms of industrial dyes. Sci Total Environ 644:1–13. https://doi.org/10.1016/j.scitotenv.2018.06.274
Del Cerro C, Erickson E, Dong T, Wong A R, Eder E K, Purvine S O, Mitchell H D, Weitz K K, Markillie L M, Burnet M. C. et al (2021) Intracellular pathways for lignin catabolism in white-rot fungi. Proc Natl Acad Sci 118(9):e2017381118. https://doi.org/10.1073/pnas.2017381118
Levasseur A, Lomascolo A, Chabrol O et al (2014) The genome of the white-rot fungus Pycnoporus cinnabarinus: a basidiomycete model with a versatile arsenal for lignocellulosic biomass breakdown. BMC Genomics 15(486). https://doi.org/10.1186/1471-2164-15-486
Ilić N, Davidović S, Milić M et al (2022) Valorization of lignocellulosic wastes for extracellular enzyme production by novel basidiomycetes: screening, hydrolysis, and bioethanol production. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-02145-x
Shankar A, Saini S, Sharma KK (2022) Fungal-integrated second-generation lignocellulosic biorefinery: utilization of agricultural biomass for co-production of lignocellulolytic enzymes, mushroom, fungal polysaccharides, and bioethanol. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-022-02969-1
Arya PS, Yagnik SM, Rajput KN, Panchal RR, Raval VH (2022) Valorization of agro-food wastes: ease of concomitant-enzymes production with application in food and biofuel industries. Bioresour Technol 361. https://doi.org/10.1016/j.biortech.2022.127738
Lestan D, Lamar RT (1996) Development of fungal inocula for bioaugmentation of contaminated soils. Appl Environ Microbiol 62(6):2045–2052
Viayaraghavan P, Kumar SJ, Arasu MV, Al-dhabi NA (2019) Simultaneous production of commercial enzymes using agro industrial residues by statistical approach. J Sci Food Agric 99:2685–2696. https://doi.org/10.1002/jsfa.9436
Matei JC, Soares M, Bonato ACH, de Freitas MPA, Helm CV, Maroldi WV et al (2020) Enzymatic delignification of sugar cane bagasse and rice husks and its effect in saccharification. Renew Energy 157:987–997. https://doi.org/10.1016/j.renene.2020.05.028
Ryvarden L (1991) Genera of polypores: nomenclature and taxonomy. Synopsis Fungorum 5:1–363
Teixeira AR (1993) Chave para identificação dos gêneros de Polyporaceae com base na morfologia do basidiocarpo. Bol Inst Bot 8:1–55
Teixeira AR (1995) Método para estudo das hifas do basidiocarpo de fungos poliporáceos. Manual do Instituto de Botânica 6:1–20
Zhang Y, Wang J, Yajun C, Zhou M, Wang W, Geng M, Xu D (2020) Comparative genomics uncovers the genetic diversity and synthetic biology of secondary metabolite production of Trametes. Mycrobiology 48(2):104–114. https://doi.org/10.1080/12298093.2020.1725361
Welti S, Moreau PA, Favel A, Courtecuisse R, Haon M, Navarro D, Taussac S, Lesage-Meessen L (2012) Molecular phylogeny of Trametes and related genera, and description of a new genus Leiotrametes. Fungal Divers 55:47–64. https://doi.org/10.1007/s13225-011-0149-2
Braga-neto, R. (2013) P. sanguineus (v1) in Biogeografia da Flora e dos Fungos do Brasil. INCT Herbário Virtual (http://biogeo.inct.florabrasil.net/proc/4582)
Scarpa JCP, Marques NP, Monteiro DA, Martins GM, Paula AV, Boscolo M, Silva R, Gomes E, Bocchini DA (2019) Saccharification of pretreated sugarcane bagasse using enzymes solution from P. sanguineus MCA 16 and cellulosic ethanol production. Ind Crops Prod 141:111795. https://doi.org/10.1016/j.indcrop.2019.111795
Lesage-Meessen L, Haon M, Uzan E, Levasseur A, Piumi F, Navarro D et al (2011) Phylogeographic relationships in the polypore fungus Pycnoporus inferred from molecular data. FEMS Microbiol Lett 325(1):37–48. https://doi.org/10.1111/j.1574-6968.2011.02412.x
Gutiérrez A, Rencoret J, Cadena EM, Rico A, Barth D, del Río JC et al (2012) Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour Technol 119:114–122. https://doi.org/10.1016/j.biortech.2012.05.112
Tomé LM, da Silva FF, Fonseca PL, Mendes-Pereira T, Azevedo VA, Brenig B, Badotti F, Góes-Neto A (2022) Hybrid assembly improves genome quality and completeness of T. villosa CCMB561 and reveals a huge potential for lignocellulose breakdown. J Fungi 8:142. https://doi.org/10.3390/jof8020142
Marzo C, Díaz AB, Blandino A (2018) Valorization of agro-industrial wastes to produce hydrolytic enzymes by fungal solid-state fermentation. Sage J 37. https://doi.org/10.1177/0734242X18798699
Yesilada O, Asma D, Cing S (2003) Decolorization of textile dyes by fungal pellets. Process Biochem 38(6):933–938. https://doi.org/10.1016/s0032-9592(02)00197-8
Mori T, Takahashi S, Soga A, Arimoto M, Kishikawa R, Yama Y et al (2023) Aerobic H2 production related to formate metabolism in white-rot fungi. Front Fungal Biol 4:1201889
Birhanli E, Yesilada O (2006) Increased production of laccase by pellets of Funalia trogii ATCC 200800 and Trametes versicolor ATCC 200801 in repeated-batch mode. Enzym Microb Technol 39(6):1286–1293. https://doi.org/10.1016/j.enzmictec.2006.03.015
Ding C, Wang X, Li M (2019) Evaluation of six white-rot fungal pretreatments on corn stover for the production of cellulolytic and ligninolytic enzymes, reducing sugars, and ethanol. Appl Microbiol Biotechnol 103(14):5641–5652. https://doi.org/10.1007/s00253-019-09884-y
Buratti S, Rinaldi F, Calleri E, Bernardi M, Oliva D, Malgaretti M, De Girolamo G, Barucco B, Girometta CE, Savino E (2023) Ganoderma resinaceum and Perenniporia fraxinea: two promising wood decay fungi for pharmaceutical degradation. J Fungi 9:555. https://doi.org/10.3390/jof9050555
Dessalew G, Beyene A, Nebiyu A, Ruelle ML (2017) Use of industrial diatomite wastes from beer production to improve soil fertility and cereal yields. J Clean Prod 157:22–29
Semiao MA, Haminiuk CWI, Maciel GM (2020) Residual diatomaceous earth as a potential and cost effective biosorbent of the azo textile dye Reactive Blue 160. J Environ Chem Eng 8(1):103617
Monclaro AV, Fontes PR, Recalde GL, Silva Júnior G, Ferreira Filho EX (2022) Evaluation of endoglucanase and xylanase production by Aspergillus tamarii cultivated in agro-industrial lignocellulosic biomasses. Folia Microbiol 7:721–732. https://doi.org/10.1007/s12223-022-00971-8
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Biz A, Farias FC, Motter FA, Paula DH, Richard P, Krieger N, Mitchell DA (2014) Pectinase activity determination: na early deceleration in the release of reducing sugars throws a spanner in th works! PLoS One 10(9). https://doi.org/10.1371/journal.pone.0109529
Mota TR, Kato CG, Peralta RA, Bracht A, De Morais GR, Baesso ML, De Souza CGM, Peralta RM (2015) Decolourization of Congo red by Ganoderma lucidum laccase: evaluation of degradation products and toxicity. Water Air Soil Pollut 226(10). https://doi.org/10.1007/s11270-015-2612-2
Rodríguez E, Pickard MA, Vazquez-Duhalt R (1999) Industrial dye decolorization by laccases from ligninolytic fungi. Curr Microbiol 38(1):27–32. https://doi.org/10.1007/PL00006767
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2(4):875–877. https://doi.org/10.1038/nprot.2007.102
Vilar DS, Fernandes CD, Nascimento VR, Torres NH, Leite MS, Bharagava RN et al (2021) Hyper-production optimization of fungal oxidative green enzymes using citrus low-cost byproduct. J Environ Chem Eng 9(1):105013
Okal EJ, Aslam MM, Karanja JK, Nyimbo WJ (2020) Mini review: Advances in understanding regulation of cellulase enzyme in white-rot basidiomycetes. Microb Pathog 147:104410
Irbe I, Elisashvili V, Asatiani MD, Janberga A, Andersone I, Andersons B et al (2014) Lignocellulolytic activity of Coniophora puteana and Trametes versicolor in fermentation of wheat bran and decay of hydrothermally modified hardwoods. Int Biodeterior Biodegradation 86:71–78. https://doi.org/10.1016/j.ibiod.2013.06.027
Falkoski DL, Guimarães VM, de Almeida MN et al (2012) Characterization of cellulolytic extract from P. sanguineus PF-2 and its application in biomass saccharification. Appl Biochem Biotechnol 166:1586–1603. https://doi.org/10.1007/s12010-012-9565-3
Christina MP, Brandon CK, Heather BM, Henrik H, Michael EH, Mats S, Jerry S, Gregg TB (2015) Fungal cellulases. Chem Rev 115(3):1308–1448. https://doi.org/10.1021/cr500351c
Rodríguez MD, Paiva IMA, Castrillo ML, Zapata PD, Villalba LL (2019) KH2PO4 improves cellulase production of Irpex lacteus and Pycnoporus sanguineus. J King Saud Univ Sci 31(4):434–444
Peng X, Su H, Mi S et al (2016) A multifunctional thermophilic glycoside hydrolase from Caldicellulosiruptor owensensis with potential applications in production of biofuels and biochemicals. Biotechnol Biofuels 9(98). https://doi.org/10.1186/s13068-016-0509-y
Schmitz K, Protzko R, Zhang L et al (2019) Spotlight on fungal pectin utilization—from phytopathogenicity to molecular recognition and industrial applications. Appl Microbiol Biotechnol 103:2507–2524. https://doi.org/10.1007/s00253-019-09622-4
Sousa EC, Uchôa-Thomaz AM, Carioca JO et al (2014) Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci Technol 34(1):135–142. https://doi.org/10.1590/S0101-20612014000100020
Spinei M, Oroian M (2023) Structural, functional, and physicochemical properties of pectin from grape pomace as affected by different extraction techniques. Int J Biol Macromol 224:739–753. https://doi.org/10.1016/j.ijbiomac.2022.10.162
Williams DL, Schückel J, Vivier MA, Buffetto F, Zietsman AJ (2019) Grape pomace fermentation and cell wall degradation by Kluyveromyces marxianus Y885. Biochem Eng J 150:107282. https://doi.org/10.1016/j.bej.2019.107282
Lara-Marquez A, Zavala-Paramo M, Lopez-Romero E, Calderon-Cortes N, Lopez-Gomez R, Conejo-Saucedo U et al (2011) Cloning and characterization of a pectin lyase gene from Colletotrichum lindemuthianum and comparative phylogenetic/structural analyses with genes from phytopathogenic and saprophytic/opportunistic microorganisms. BMC Microbiol 11:260
Shrestha S, Rahman MS, Qin W (2021) New insights in pectinase production development and industrial applications. Appl Microbiol Biotechnol 105:9069–9087 https://doi-org.ez48.periodicos.capes.gov.br/10.1007/s00253-021-11705-0
González-Bautista E, Alarcón-Gutierrez E, Dupuy N, Gaime-Perraud I, Ziarelli F, Farnet-Da-Silva AM (2020) Influence of yeast extract enrichment and Pycnoporus sanguineus inoculum on the dephenolisation of sugar-cane bagasse for production of second-generation ethanol. Fuel 260:116370
Collins PJ, Dobson ADW (1997) Regulation of laccase gene transcription in Trametes versicolor. Appl Environ Microbiol 63:3444–3450
Valle JS, Vandenberghe LPS, Santana TT, Almeida PH, Pereira AM, Linde GA et al (2014) Optimum conditions for inducing laccase production in Lentinus crinitus. Genet Mol Res 13(4):8544–8551
Camarero S, Pardo I, Cañas AI, Molina P, Record E, Martínez AT et al (2012) Engineering platforms for directed evolution of laccase from Pycnoporus cinnabarinus. Appl Environ Microbiol 78(5):1370–1384
Umar A, Ahmed S (2022) Optimization, purification and characterization of laccase from Ganoderma leucocontextum along with its phylogenetic relationship. Sci Rep 12(1):2416. https://doi.org/10.1038/s41598-022-06111-z
Coniglio RO, Diaz GV, Baruá RC, Alberto E, Zapata PD (2021) Enzyme-assisted extraction of phenolic compounds and proteins from sugarcane bagasse using a low0cost cocktail from Auricularia fuscosuccinea. Int J Food Sci Technol 57:1114–1121. https://doi.org/10.1111/ijfs.15477
Pattnaik B, Sarangi PK, Jena PK, Sahoo HP, Bhatia L (2021) Production of phenolic flavoring compounds from sugarcane bagasse by Lactobacillus acidophilus MTCC 10307. Arch Microbiol 204(23). https://doi.org/10.1007/s00203-021-02655-2
Gambato G, Todescato K, Pavão EM, Scortegagna A, Fontana RC, Salvador M, Camassola M (2016) Evaluation of productivity and antioxidant profile of solid-state cultivated macrofungi Pleurotus albidus and Pycnoporus sanguineus. Bioresour Technol 207:46–51. https://doi.org/10.1016/j.biortech.2016.01.121
Zhang L, Deng Z, Qiu T, Yang W, Zhu F, Ye X (2023) Characterisation of a laccase isolated from Trametes hirsuta and its application in the oligomerisation of phenolic compounds. Fungal Biol 127(1-2):872–880. https://doi.org/10.1016/j.funbio.2022.11.005
da Silva EA, Mendes TD, Pacheco TF et al (2022) Colonization of oil palm empty fruit bunches by basidiomycetes from the Brazilian cerrado: Enzyme production. Energy Sci Eng 10:1189–1201. https://doi.org/10.1002/ese3.1093
Yoon LW, Ngoh GC, Chua AS, Patah MF, Teoh WH (2019) Process intensification of cellulase and bioethanol production from sugarcane bagasse via an integrated saccharification and fermentation process. Chem Eng Process Process Intensif 142:107528. https://doi.org/10.1016/j.cep.2019.107528
Mussatto SI, Machado EMS, Martins S et al (2011) Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol 4:661–672. https://doi.org/10.1007/s11947-011-0565-z
Ferreira DSS, Santana CS, Santana IB, Araújo JSC, Souza BC, Leite FHA (2021) Functional annotation and comparative modeling of ligninolytic enzymes from T. villosa (SW.) Kreisel for biotechnological applications. J Biomol Struct Dyn 40(14):6330–6339. https://doi.org/10.1080/07391102.2021.1883479
Alexopoulos CJ, Mims CW, Blackwell M (1996) Phylum Basidiomycota order Aphyllophorales, Polypores, Chantharelles, tooth fungi, coral fungi and corticioids. In: Micologia introdutória, 4ª edn. John Wiley & Sons, Inc.
Pineda-Insuasti JA, Gómez-Andrade WE, Duarte-Trujillo AS, Soto-Arroyave CP, Pineda-Soto CA, Fierro-Ramos FJ, Mora-Muñoz ES, Álvarez-Ramos SE (2017) Producción de Pycnoporus spp. y sus metabolitos secundarios: Una revisión. ICIDCA Sobre los Derivados de la Caña de Azúcar 51(2):60–69 http://www.redalyc.org/articulo.oa?id=223154251010
STATSOFT INC. (2007) Statistica data analysis system version 8.0. Tulsa: Statsoft Inc. 91(3):339-341. https://doi.org/10.1007/s10182-007-0038-x
De Paula NM, da Silva K, Brugnari T, Haminiuk CWI, Maciel GM (2022) Biotechnological potential of fungi from a mangrove ecosystem: enzymes, salt tolerance and decolorization of a real textile effluent. Microbiol Res 254:126899
Eichlerová I, Homolka L, Benada O, Kofroňová O, Hubálek T, Nerud F (2007) Decolorization of orange G and remazol brilliant blue R by the white rot fungus Dichomitus squalens: toxicological evaluation and morphological study. Chemosphere 69(5):795–802. https://doi.org/10.1016/j.chemosphere.2007.04.083
Stafussa AP, Maciel GM, da Silva Anthero AG, da Silva MV, Zielinski AAF, Haminiuk CWI (2016) Biosorption of anthocyanins from grape pomace extracts by waste yeast: kinetic and isotherm studies. J Food Eng 169:53–60
Tapangnoi P, Sae-Oui P, Naebpetch W, Siriwong C (2022) Preparation of purified Spent coffee ground and its reinforcement in natural rubber composite. Arab J Chem 14. https://doi.org/10.1016/j.arabjc.2022.103917
Sousa EC, Uchôa-Thomaz AMA, Carioca JOB, Morais SM, Lima A et al (2014) Chemical composition and bioactive compounds of grape pomace (Vitis vinífera L), Benitaka Variety, grown in the semiarid region of Northheast Brazil. Food Sci Technol 34(1):135–142. https://doi.org/10.1590/S0101-20612014000100020
Coelho CCS, Michelin M, Cerqueira MA et al (2018) Cellulose nanocrystals from grape pomace: production, properties and cytotoxicity assessment. Carbohydr Polym 192:327–336. https://doi.org/10.1016/j.carbpol.2018.03.023
Abouelenein D, Mustafa AM, Caprioli G, Ricciutelli M, Sagratini G, Vittori S (2023) Phenolic and nutritional profiles, and antioxidant activity of grape pomaces and seeds from Lacrima di Morro d’Alba and Verdicchio varieties. Food. Bioscience 53 https://doi-org.ez48.periodicos.capes.gov.br/10.1016/j.fbio.2023.102808
Rodríguez-Ramos F, Cañas-Sarazúa R, Briones-Labarca V (2022) Pisco grape pomace: iron/Copper speciation and antioxidant properties, towards their comprehensive utilization. Food Biosci 47. https://doi.org/10.1016/j.fbio.2022.101781
Martin N, Souza SR, Silva R, Gomes E (2004) Pectinase production by fungal strains in solid-state fermentation using agro-industrial bioproduct. Braz Arch Biol Technol 47(5):813–819. https://doi.org/10.1590/S1516-89132004000500018
Dimopoulo M, Kontogiorgos V (2019) Soluble dietary fibres from sugarcane bagasse. Int J Food Sci Technol 55:1943–1949. https://doi.org/10.1111/ijfs.14445
Filippi K, Georka N, Alexandri M, Papapostolou H, Koutinas A (2021) Valorisation of grape stalks and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind Crop Prod 168. https://doi.org/10.1016/j.indcrop.2021.113578
Singh SP, Jawaid M, Chandrasekar M, Senthilkumar K, Yadav B, Saba N, Siengchin S (2021) Sugarcane wastes into commercial product: processing metods, production Optimization and challenges. J Clean Prod 328. https://doi.org/10.1016/j.jclepro.2021.129453
Wani AK, Rahayu F, Fauziah L, Suhara C (2023) Advances in safe processing of Sugarcane and bagasse for the generation of biofuels and bioactive compounds. J Agric Food Res 12 https://doi-org.ez48.periodicos.capes.gov.br/10.1016/j.jafr.2023.100549
Nakamura Y, Ono Y, Saito T, Isogai A (2019) Characterization of cellulose microfibrils, cellulose molecules, and hemicelluloses in buckwheat and rice husks. Cellulose 26:6529–6541. https://doi.org/10.1007/s10570-019-02560-4
Wisetkomolmat J, Arjin C, Hongsibsong S et al (2023) Antioxidant activities and characterization of polyphenols from selected northern Thai rice husks: relation with seed attributes. Rice Sci 30:148–159 https://doi-org.ez48.periodicos.capes.gov.br/10.1016/j.rsci.2023.01.007
Wu J, Elliston A, Gall GL, Colquhoun IJ et al (2018) Optimising conditions for bioetanol production from rice husk and rice straw: effects of pre-treatment on liquor composition and fermentation inhibitors. Biotechnol Biofuels 11(62). https://doi.org/10.1186/s13068-018-1062-7
Carvalho AFA, Figueiredo FC, Campioni TS, Pastore GM, Neto PO (2020) Improvement of some chemical and biological methods for the eficiente production of xylanases, xylooligosaccharides and linocellulose from sugar cane bagasse. Biomass Bioenergy 143. https://doi.org/10.1016/j.biombioe.2020.105851
Madrid R, Margarido F, Nogueira CA (2012) Valorisation of rice husks by chemical and therma treatments. Mater Sci Forum 730-732:659–664. https://doi.org/10.4028/www.scientific.net/MSF.730-732.659
Xu F, Zhang S, Waterhouse GI, Zhou T, Du Y, Sun-Waterhouse D, Wu P (2022) Yeast fermentation of apple and grape pomaces affects subsequent aqueous pectin extraction: composition, structure, functional and antioxidant properties of pectins. Food Hydrocoll 133. https://doi.org/10.1016/j.foodhyd.2022.107945
Coniglio RO, Díaz GV, Fonseca MI, Castrillo ML, Piccinni FE, Villalba LL et al (2020) Enzymatic hydrolysis of barley straw for biofuel industry using a novel strain of T. villosa from Paranaense rainforest. Prep Biochem Biotechnol 50(8):753–762. https://doi.org/10.1080/10826068.2020.1734941
Carvalho HAS, Ribeiro LF, Pirovani CP, Gramacho KP, Micheli F (2013) Activity of polygalacturonases from Moniliophthora perniciosa depends on fungus culture conditions and is enhanced by Theobroma cacao extracts. Physiological and Molecular Plant Pathology 83:40–50. https://doi.org/10.1016/j.pmpp.2013.04.001
Acknowledgements
We thank the Federal University of Technology – Paraná and the Coordination for the Improvement of Higher Education Personnel for scholarships granted to Thaiany da Silva Soares, the National Council for Scientific and Technological Development (CNPq) for C.W.I. Haminiuk and G.M. Maciel research productivity grants, the Multi User Laboratory of Equipment and Environmental Analysis (LAMEAA) situated at Federal University of Technology-Paraná for making available all the instrumentation which benefited this research.
Funding
This work was supported by the Federal University of Technology – Paraná (scholarship for T. S. Soares, and resources for the Biotechnology Laboratory), the Coordination for the Improvement of Higher Education Personnel (scholarship for T. S. Soares), and the National Council for Scientific and Technological Development (CNPq) (research productivity grants for C.W.I. Haminiuk and G. M. Maciel).
Author information
Authors and Affiliations
Contributions
Thaiany da Silva Soares: Formal analysis, Investigation, Writing – original draft; Charles Windson Isidoro Haminiuk: Resources, Writing – review and editing; Giselle Maria Maciel: Conceptualization, Methodology, Resources, Writing – review and editing, Supervision.
Corresponding author
Ethics declarations
Ethical approval
This declaration is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1082 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva Soares, T., Haminiuk, C.W.I. & Maciel, G.M. Fungal biorefinery for simultaneous production of enzymes and bioconversion of agro-industrial residues into renewable sugars and phenolic compounds. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04706-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04706-8