Abstract
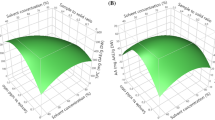
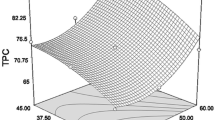
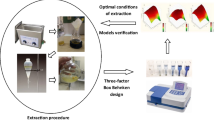
Response surface methodology was used to optimize the ultrasonic-assisted extraction (UAE) of total flavonoids (TF) and total phenols (TP) from Berberis kongboensis fruit. Single-factor experiments were conducted with the ultrasonication power, liquid–solid ratio, ethanol concentration, extraction temperature, and extraction time. A Plackett–Burman design was used to identify the main factors affecting the extraction rates of TF and TP. A Box–Behnken design was used to further optimize the ethanol concentration, solvent/sample ratio, and extraction temperature. Under the optimum conditions, the ethanol concentration of 34.67%, solvent/sample ratio of 38.44 mL/g, and extraction temperature of 46.53 °C, the yields of TF and TP were 107.92 mg rutin equivalents/g and 4.86 mg gallic acid equivalents/g, respectively. The extract from UAE had a better yield and higher antioxidant activities in vitro than that obtained by conventional solvent extraction. The B. kongboensis fruit is rich in flavonoids and phenolics, and it has potential application value in the food industry.




Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- UAE:
-
Ultrasound-assisted extraction
- CSE:
-
Conventional solvent extraction
- TF:
-
Total flavonoids
- TP:
-
Total phenolics
- RT:
-
Rutin
- GA:
-
Gallic acid
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- FRAP:
-
Ferric ion reducing antioxidant power
- TRAP:
-
Total radical-trapping antioxidant parameter
References
Ying J (2011) Berberis Linnaeus (Sp. Pl. 1: 330. 1753.). Flora of China 19:715–771
Radziejewska Kubzdela E, Szwengiel A, Ratajkiewicz H, Nowak K (2020) Effect of ultrasound, heating and enzymatic pre-treatment on bioactive compounds in juice from Berberis amurensis Rupr. Ultrason Sonochem 63:104971. https://doi.org/10.1016/j.ultsonch.2020.104971
Emamat H, Asadian S, Zahedmehr A, Ghanavati M, Nasrollahzadeh J (2020) The effect of barberry (Berberis vulgaris) consumption on flow-mediated dilation and inflammatory biomarkers in patients with hypertension: a randomized controlled trial. Phytother Res 35:2607–2615. https://doi.org/10.1002/ptr.7000
Afsharinasab M, Mohammad-Sadeghipour M, Reza Hajizadeh M, Khoshdel A, Mirzaiey V, Mahmoodi M (2020) The effect of hydroalcoholic Berberis integerrima fruits extract on the lipid profile, antioxidant parameters and liver and kidney function tests in patients with nonalcoholic fatty liver disease. Saudi J Biol Sci 27:2031–2037. https://doi.org/10.1016/j.sjbs.2020.04.037
Calderón Reyes C, Pezoa RS, Leal P, Ribera-Fonseca A, Cáceres C, Riquelme I, Zambrano T, Peña D, Alberdi M, Reyes-Díaz M (2020) Anthocyanin-rich extracts of calafate (Berberis microphylla G. Forst.) fruits decrease in vitro viability and migration of human gastric and gallbladder cancer cell lines. J Soil Sci Plant Nut 20:1891–1903. https://doi.org/10.1007/s42729-020-00260-8
El Fakir L, Bouothmany K, Alotaibi A, Bourhia M, Ullah R, Zahoor S, El Mzibri M, Gmouh S, Alaoui T, Zaid A, Benbacer L (2021) Antioxidant and understanding the anticancer properties in human prostate and breast cancer cell lines of chemically characterized methanol extract from Berberis hispanica Boiss. & Reut. Appl Sci 11:3510–3521. https://doi.org/10.3390/app11083510
Ilyas S, Tabasum R, Iftikhar A, Nazir M, Hussain A, Hussain A, Ali MS, Saleem F, Saleem U, Froeyen M, Abdullah I, Mirza MU, Ahmad S (2021) Effect of Berberis vulgaris L. root extract on ifosfamide-induced in vivo toxicity and in vitro cytotoxicity. Sci Rep 11:1708–1721. https://doi.org/10.1038/s41598-020-80579-5
Mustafa K, Mohamed H, Shah AM, Yu S, Akhlaq M, Xiao H, Li S, Naz T, Nosheen S, Bai X, Song Y (2020) In vitro anticancer potential of Berberis lycium Royle extracts against human hepatocarcinoma (HepG2) cells. Biomed Res Int 2020:8256809. https://doi.org/10.1155/2020/8256809
Landry KB, Azam S, Rehman S, Tariq S, Iqbal B, Abbas M, Lembè D (2021) Phytochemical analysis of Berberis lyceum methanolic extract and its antiviral activity through the restoration of MAPK signaling pathway modulated by HCV NS5A. Asian Pac J Trop Bio 11:132–140. https://doi.org/10.4103/2221-1691.306133
Sabahi Z, Farmani F, Soltani F, Moein M (2018) DNA protection, antioxidant and xanthine oxidase inhibition activities of polyphenol-enriched fraction of Berberis integerrima Bunge fruits. Iran J Basic Med Sci 21:411–416. https://doi.org/10.22038/IJBMS.2018.26563.6506
Bisht A, Giri L, Belwal T, Pandey A, Bahukhandi A, Bhatt ID, Rawal Ranbeer S (2021) In vitro propagation and antioxidant potential of Berberis asiatica from Western Himalaya. Plant Biosys Int J Dealing Aspects of Plant Biol 156: 490-496. https://doi.org/10.1080/11263504.2021.1887953
Jaberi R, Kaban G, Kaya M (2020) The effect of barberry (Berberis vulgaris L.) extract on the physicochemical properties, sensory characteristics, and volatile compounds of chicken frankfurters. J Food Process Pres 44:14501–14511. https://doi.org/10.1111/jfpp.14501
Soto Covasich J, Reyes Farias M, Torres RF, Vasquez K, Duarte L, Quezada J, Jimenez P, Pino MT, Garcia-Nannig L, Mercado L, Garcia-Diaz DF (2020) A polyphenol-rich calafate (Berberis microphylla) extract rescues glucose tolerance in mice fed with cafeteria diet. J Funct Foods 67:103856. https://doi.org/10.1016/j.jff.2020.103856
Tahmasebi L, Zakerkish M, Golfakhrabadi F, Namjoyan F (2019) Randomised clinical trial of Berberis vulgaris root extract on glycemic and lipid parameters in type 2 diabetes mellitus patients. Eur J Integr Med 32:100998. https://doi.org/10.1016/j.eujim.2019.100998
Xu T, Ge Y, Du H, Li Q, Xu X, Yi H, Wu X, Kuang T, Fan G, Zhang Y (2021) Berberis kansuensis extract alleviates type 2 diabetes in rats by regulating gut microbiota composition. J Ethnopharmacol 273:113995. https://doi.org/10.1016/j.jep.2021.113995
Nazir N, Rahman A, Uddin F, Khan Khalil AA, Zahoor M, Nisar M, Ullah S, Ullah R, Ezzeldin E, Mostafa GAE (2021) Quantitative ethnomedicinal status and phytochemical analysis of Berberis lyceum Royle. Agronomy 11:130–142. https://doi.org/10.3390/agronomy11010130
Çakır Ö, Karabulut A (2020) Comparison of two wild-grown Berberis varieties based on biochemical characterization. J Food Process Pres 44:14844–14854. https://doi.org/10.1111/jfpp.14844
Dong ZY, Zeng QH, Wei L, Guo X, Sun Y, Meng FC, Wang GW, Lan XZ, Liao ZH, Chen M (2022) Berberisides A-D: three novel prenylated benzoic acid derivatives and a clerodane glycoside from Berberis tsarica aherndt. Nat Prod Res 36:1996–2001. https://doi.org/10.1080/14786419.2020.1839460
Arena ME, Giordani E, Bustamante G, Radice S (2021) Variability in fruit traits and anthocyanin content among and within populations of underutilized Patagonian species Berberis microphylla G. Forst. J Berry Research 11:33–50. https://doi.org/10.3233/JBR-200599
Sharifi A, Niakousari M, Mortazavi SA, Elhamirad AH (2019) High-pressure CO2 extraction of bioactive compounds of barberry fruit (Berberis vulgaris): process optimization and compounds characterization. J Food Meas Charact 13:1139–1146. https://doi.org/10.1007/s11694-018-00029-9
Hostalkova A, Marikova J, Opletal L, Korabecny J, Hulcova D, Kunes J, Novakova L, Perez DI, Jun D, Kucera T, Andrisano V, Siatka T, Cahlikova L (2019) Isoquinoline alkaloids from Berberis vulgaris as potential lead compounds for the treatment of Alzheimer’s disease. J Nat Prod 82:239–248. https://doi.org/10.1021/acs.jnatprod.8b00592
Rahim BZ (2019) Nutritional and phytochemical screening of wild fruit of Berberis baluchistanica – an endemic species To Pakistan. Appl Ecol Env Res 17:12697–12707. https://doi.org/10.15666/aeer/1706_1269712707
Cascaes Teles AS, Hidalgo Chávez DW, Zarur Coelho MA, Rosenthal A, Fortes Gottschalk LM, Tonon RV (2021) Combination of enzyme-assisted extraction and high hydrostatic pressure for phenolic compounds recovery from grape pomace. J Food Eng 288:110128. https://doi.org/10.1016/j.jfoodeng.2020.110128
Zhou L, Luo S, Li J, Zhou Y, Wang X, Kong Q, Chen T, Feng S, Yuan M, Ding C (2021) Optimization of the extraction of polysaccharides from the shells of Camellia oleifera and evaluation on the antioxidant potential in vitro and in vivo. J Funct Foods 86:104678. https://doi.org/10.1016/j.jff.2021.104678
Zheng J, Li H, Wang D, Li R, Wang S, Ling B (2021) Radio frequency assisted extraction of pectin from apple pomace: process optimization and comparison with microwave and conventional methods. Food Hydrocolloids 121:107031. https://doi.org/10.1016/j.foodhyd.2021.107031
Ingrando I, Rivoira L, Castiglioni M, Tumiatti V, Lenzi F, Pagliano A, Bruzzoniti MC (2022) Microwave-assisted extraction and gas chromatographic determination of thirty priority micropollutants in biowaste fraction derived from municipal solid waste for material recovery in the circular-economy approach. Talanta 241:123268. https://doi.org/10.1016/j.talanta.2022.123268
Alves TP, Triques CC, Palsikowski PA, da Silva C, Fiorese ML, da Silva EA, Fagundes Klen MR (2022) Improved extraction of bioactive compounds from Monteverdia aquifolia leaves by pressurized-liquid and ultrasound-assisted extraction: yield and chemical composition. J Supercrit Fluid 181:105468. https://doi.org/10.1016/j.supflu.2021.105468
Tejedor Calvo E, Garcia Barreda S, Sanchez S, Morte A, Siles Sanchez MLN, Soler Rivas C, Santoyo S, Marco P (2022) Application of pressurized liquid extractions to obtain bioactive compounds from Tuber aestivum and Terfezia claveryi. Foods 11:298–316. https://doi.org/10.3390/foods11030298
Roselló-Soto E, Galanakis CM, Brnčić M, Orlien V, Trujillo FJ, Mawson R, Knoerzer K, Tiwari BK, Barba FJ (2015) Clean recovery of antioxidant compounds from plant foods, byproducts and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci Technol 42:134–149. https://doi.org/10.1016/j.tifs.2015.01.002
Wijngaard H, Hossain MB, Rai DK, Brunton N (2012) Techniques to extract bioactive compounds from food by-products of plant origin. Food Res Int 46:505–513. https://doi.org/10.1016/j.foodres.2011.09.027
Chen XQ, Li ZH, Liu LL, Wang H, Yang SH, Zhang JS, Zhang Y (2021) Green extraction using deep eutectic solvents and antioxidant activities of flavonoids from two fruits of Rubia species. Lwt 148:111708. https://doi.org/10.1016/j.lwt.2021.111708
Chen XQ, Li ZH, Wang ZJ, Liu LL, Sun TT, Ma JZ, Zhang Y (2020) Ultrasound-assisted extraction of total anthocyanins from Rubia sylvatica Nakai fruit and radical scavenging activity of the extract. Ind Crop Prod 150:112420. https://doi.org/10.1016/j.indcrop.2020.112420
Li C, Chen S, Sha J, Cui J, He J, Fu J, Shen Y (2021) Extraction and purification of total flavonoids from Eupatorium lindleyanum DC. and evaluation of their antioxidant and enzyme inhibitory activities. Food Sci Nutr 9:2349–2363. https://doi.org/10.1002/fsn3.1999
Belwal T, Dhyani P, Bhatt ID, Rawal RS, Pande V (2016) Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem 207:115–124. https://doi.org/10.1016/j.foodchem.2016.03.081
Irakli M, Chatzopoulou P, Ekateriniadou L (2018) Optimization of ultrasound-assisted extraction of phenolic compounds: oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind Crop Prod 124:382–388. https://doi.org/10.1016/j.indcrop.2018.07.070
Xu J, Wang W, Liang H, Zhang Q, Li Q (2015) Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma longa L. using response surface methodology. Ind Crop Prod 76:487–493. https://doi.org/10.1016/j.indcrop.2015.07.025
Liu HM, Lei SN, Tang W, Xun MH, Zhao ZW, Cheng MY, Zhang XD,WangW (2022) Optimization of ultrasound-assisted cellulase extraction from Nymphaea hybrid flower and biological activities: antioxidant activity, protective effect against ROS oxidative damage in HaCaT cells and inhibition of melanin production in B16 cells. Molecules 27:1914. https://doi.org/10.3390/molecules27061914.
Zhong XY, Zhang SY, Wang H, Yang JY, Li L, Zhu J, Liu YJ (2022) Ultrasound-alkaline combined extraction improves the release of bound polyphenols from pitahaya (Hylocereus undatus ‘Foo-Lon’) peel: composition, antioxidant activities and enzyme inhibitory activity. Ultrason Sonochem 90:106213. https://doi.org/10.1016/j.ultsonch.2022.106213
Yang J, Li NN, Wang CY, Chang T, Jiang HC (2021) Ultrasound-homogenization-assisted extraction of polyphenols from coconut mesocarp: optimization study. Ultrason Sonochem 78:105739. https://doi.org/10.1016/j.ultsonch.2021.105739
Yan Yl YCH, Chen J, Li XX, Wang W, Li SQ (2011) Ultrasonic-assisted extraction optimized by response surface methodology, chemical composition and antioxidant activity of polysaccharides from Tremella mesenterica. Carbohyd Polym 83:217–224. https://doi.org/10.1016/j.carbpol.2010.07.045
Espada Bellido E, Ferreiro Gonzalez M, Carrera C, Palma M, Barroso CG, Barbero GF (2017) Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem 219:23–32. https://doi.org/10.1016/j.foodchem.2016.09.122
Meir S, Kanner J, Akiri B, Philosoph Hadas S (1995) Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agr Food Chem 43:1813–1819. https://doi.org/10.1021/jf00055a012
Wu Q, Yuan RY, Feng CY, Li SS, Wang LS (2019) Analysis of polyphenols composition and antioxidant activity assessment of Chinese dwarf cherry (Cerasus humilis (Bge.) Sok.). Nat Prod Commun 14:1–10. https://doi.org/10.1177/1934578X19856509
Yin DD, Yuan RY, Wu Q, Li SS, Shao S, Xu YJ, Hao XH, Wang LS (2015) Assessment of flavonoids and volatile compounds in tea infusions of water lily flowers and their antioxidant activities. Food Chem 187:20–28. https://doi.org/10.1016/j.foodchem.2015.04.032
Acknowledgements
This work was supported by the Natural Science Foundation of Heilongjiang Province (Grant No. LH2020C036), the Forestry Science Discipline Innovation Team Construction Project of Tibet Agriculture & Animal Husbandry University (Grant No. ZangCaiYuZhi 2020-001), the Central Government Guidance Local Regional Innovation Systems Construction Program (Grant No. XZ202201YD0025C), and the 111 Project (Grant No. B20088). We thank Gabrielle David, PhD, for editing the English text of a draft of this manuscript.
Funding
Natural Science Foundation of Heilongjiang Province (Grant No. LH2020C036).
Forestry Science Discipline Innovation Team Construction Project of Tibet Agriculture & Animal Husbandry University (Grant No. ZangCaiYuZhi 2020-001).
Central Government Guidance Local Regional Innovation Systems Construction Program (Grant No. XZ202201YD0025C).
111 Project (Grant No. B20088).
Author information
Authors and Affiliations
Contributions
Xiaoqiang Chen: conceptualization, data curation, formal analysis, investigation, methodology, visualization, project administration, and writing—original draft
Shihan Yang: formal analysis, methodology, resources, and writing—review and editing. Hong Yang: conceptualization, methodology, Resources, Supervision, Funding acquisition. Jinshan Zhang: data curation and methodology. Yuyuan Huang: visualization and writing—review and editing. Ying Zhang: conceptualization, data curation, methodology, writing—review and editing, supervision, and funding acquisition
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Yang, S., Yang, H. et al. Extraction of flavonoids and phenolics from Berberis kongboensis fruit. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03906-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03906-6